Atrial natriuretic peptide regulates Ca channel in early developmental cardiomyocytes
- PMID: 20107504
- PMCID: PMC2809742
- DOI: 10.1371/journal.pone.0008847
Atrial natriuretic peptide regulates Ca channel in early developmental cardiomyocytes
Abstract
Background: Cardiomyocytes derived from murine embryonic stem (ES) cells possess various membrane currents and signaling cascades link to that of embryonic hearts. The role of atrial natriuretic peptide (ANP) in regulation of membrane potentials and Ca(2+) currents has not been investigated in developmental cardiomyocytes.
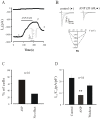
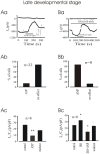
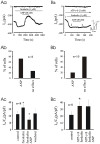
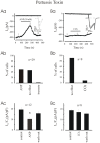
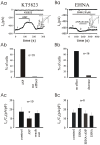
Methodology/principal findings: We investigated the role of ANP in regulating L-type Ca(2+) channel current (I(CaL)) in different developmental stages of cardiomyocytes derived from ES cells. ANP decreased the frequency of action potentials (APs) in early developmental stage (EDS) cardiomyocytes, embryonic bodies (EB) as well as whole embryo hearts. ANP exerted an inhibitory effect on basal I(CaL) in about 70% EDS cardiomyocytes tested but only in about 30% late developmental stage (LDS) cells. However, after stimulation of I(CaL) by isoproterenol (ISO) in LDS cells, ANP inhibited the response in about 70% cells. The depression of I(CaL) induced by ANP was not affected by either Nomega, Nitro-L-Arginine methyl ester (L-NAME), a nitric oxide synthetase (NOS) inhibitor, or KT5823, a cGMP-dependent protein kinase (PKG) selective inhibitor, in either EDS and LDS cells; whereas depression of I(CaL) by ANP was entirely abolished by erythro-9-(2-Hydroxy-3-nonyl) adenine (EHNA), a selective inhibitor of type 2 phosphodiesterase(PDE2) in most cells tested. CONCLUSION/SIGNIFICANCES: Taken together, these results indicate that ANP induced depression of action potentials and I(CaL) is due to activation of particulate guanylyl cyclase (GC), cGMP production and cGMP-activation of PDE2 mediated depression of adenosine 3', 5'-cyclic monophophate (cAMP)-cAMP-dependent protein kinase (PKA) in early cardiomyogenesis.
Conflict of interest statement
Figures


References
-
- Nilius B, Boldt W, Benndorf K. Properties of aconitine-modified sodium channels in single cells of mouse ventricular myocardium. Gen Physiol Biophys. 1986;5:473–484. - PubMed
-
- Kohya T, Tomita F, Itoh K, Suzuki Y, Kawabata N, et al. Silent myocardial ischemia during Holter monitoring in ischemic heart disease. Jpn Circ J. 1989;53:1399–1406. - PubMed
-
- Semmekort B, Guignard JP. Atrial natriuretic peptide during early human development. Biol Neonate. 1991;60:341–349. - PubMed
-
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. - PubMed
-
- Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, et al. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

