Temporal and spatial requirements of unplugged/MuSK function during zebrafish neuromuscular development
- PMID: 20107509
- PMCID: PMC2809748
- DOI: 10.1371/journal.pone.0008843
Temporal and spatial requirements of unplugged/MuSK function during zebrafish neuromuscular development
Abstract
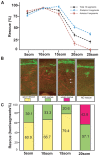
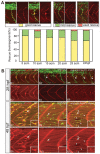
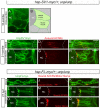
One of the earliest events in neuromuscular junction (NMJ) development is the accumulation of acetylcholine receptor (AChR) at the center of muscle cells. The unplugged/MuSK (muscle specific tyrosine kinase) gene is essential to initiate AChR clustering but also to restrict approaching growth cones to the muscle center, thereby coordinating pre- and postsynaptic development. To determine how unplugged/MuSK signaling coordinates these two processes, we examined the temporal and spatial requirements of unplugged/MuSK in zebrafish embryos using heat-shock inducible transgenes. Here, we show that despite its expression in muscle cells from the time they differentiate, unplugged/MuSK activity is first required just prior to the appearance of AChR clusters to simultaneously induce AChR accumulation and to guide motor axons. Furthermore, we demonstrate that ectopic expression of unplugged/MuSK throughout the muscle membrane results in wildtype-like AChR prepattern and neuromuscular synapses in the central region of muscle cells. We propose that AChR prepatterning and axonal guidance are spatio-temporally coordinated through common unplugged/MuSK signals, and that additional factor(s) restrict unplugged/MuSK signaling to a central muscle zone critical for establishing mid-muscle synaptogenesis.
Conflict of interest statement
Figures



References
-
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. - PubMed
-
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. - PubMed
-
- Witzemann V. Development of the neuromuscular junction. Cell Tissue Res. 2006;326:263–271. - PubMed
-
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

