TBCD links centriologenesis, spindle microtubule dynamics, and midbody abscission in human cells
- PMID: 20107510
- PMCID: PMC2809749
- DOI: 10.1371/journal.pone.0008846
TBCD links centriologenesis, spindle microtubule dynamics, and midbody abscission in human cells
Abstract
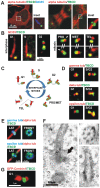
Microtubule-organizing centers recruit alpha- and beta-tubulin polypeptides for microtubule nucleation. Tubulin synthesis is complex, requiring five specific cofactors, designated tubulin cofactors (TBCs) A-E, which contribute to various aspects of microtubule dynamics in vivo. Here, we show that tubulin cofactor D (TBCD) is concentrated at the centrosome and midbody, where it participates in centriologenesis, spindle organization, and cell abscission. TBCD exhibits a cell-cycle-specific pattern, localizing on the daughter centriole at G1 and on procentrioles by S, and disappearing from older centrioles at telophase as the protein is recruited to the midbody. Our data show that TBCD overexpression results in microtubule release from the centrosome and G1 arrest, whereas its depletion produces mitotic aberrations and incomplete microtubule retraction at the midbody during cytokinesis. TBCD is recruited to the centriole replication site at the onset of the centrosome duplication cycle. A role in centriologenesis is further supported in differentiating ciliated cells, where TBCD is organized into "centriolar rosettes". These data suggest that TBCD participates in both canonical and de novo centriolar assembly pathways.
Conflict of interest statement
Figures





Similar articles
-
Emerging roles for tubulin folding cofactors at the centrosome.Commun Integr Biol. 2010 Jul;3(4):306-8. doi: 10.4161/cib.3.4.11976. Commun Integr Biol. 2010. PMID: 20798813 Free PMC article.
-
Cep57 protein is required for cytokinesis by facilitating central spindle microtubule organization.J Biol Chem. 2013 May 17;288(20):14384-14390. doi: 10.1074/jbc.M112.441501. Epub 2013 Apr 8. J Biol Chem. 2013. PMID: 23569207 Free PMC article.
-
SAS-6 Association with γ-Tubulin Ring Complex Is Required for Centriole Duplication in Human Cells.Curr Biol. 2020 Jun 22;30(12):2395-2403.e4. doi: 10.1016/j.cub.2020.04.036. Epub 2020 May 21. Curr Biol. 2020. PMID: 32442461
-
Centrosome maturation - in tune with the cell cycle.J Cell Sci. 2022 Jan 15;135(2):jcs259395. doi: 10.1242/jcs.259395. Epub 2022 Jan 28. J Cell Sci. 2022. PMID: 35088834 Review.
-
Centrosome inheritance after fertilization and nuclear transfer in mammals.Adv Exp Med Biol. 2007;591:58-71. doi: 10.1007/978-0-387-37754-4_4. Adv Exp Med Biol. 2007. PMID: 17176554 Review.
Cited by
-
Nucleotide Binding to ARL2 in the TBCD∙ARL2∙β-Tubulin Complex Drives Conformational Changes in β-Tubulin.J Mol Biol. 2017 Nov 24;429(23):3696-3716. doi: 10.1016/j.jmb.2017.09.016. Epub 2017 Sep 29. J Mol Biol. 2017. PMID: 28970104 Free PMC article.
-
The dual role of fission yeast Tbc1/cofactor C orchestrates microtubule homeostasis in tubulin folding and acts as a GAP for GTPase Alp41/Arl2.Mol Biol Cell. 2013 Jun;24(11):1713-24, S1-8. doi: 10.1091/mbc.E12-11-0792. Epub 2013 Apr 10. Mol Biol Cell. 2013. PMID: 23576550 Free PMC article.
-
Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy.Int J Mol Sci. 2023 Nov 16;24(22):16400. doi: 10.3390/ijms242216400. Int J Mol Sci. 2023. PMID: 38003592 Free PMC article.
-
Making the final cut - mechanisms mediating the abscission step of cytokinesis.ScientificWorldJournal. 2010 Jul 19;10:1424-34. doi: 10.1100/tsw.2010.129. ScientificWorldJournal. 2010. PMID: 20661535 Free PMC article. Review.
-
Cortical atrophy and hypofibrinogenemia due to FGG and TBCD mutations in a single family: a case report.BMC Med Genet. 2018 May 16;19(1):80. doi: 10.1186/s12881-018-0597-6. BMC Med Genet. 2018. PMID: 29769041 Free PMC article.
References
-
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, et al. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. - PubMed
-
- Lewis SA, Tian G, Cowan NJ. The alpha- and beta-tubulin folding pathways. Trends Cell Biol. 1997;12:479–484. - PubMed
-
- López-Fanarraga M, Avila J, Guasch A, Coll M, Zabala JC. Review: postchaperonin tubulin folding cofactors and their role in microtubule dynamics. J Struct Biol. 2001;135:219–229. - PubMed
-
- Szymanski D. Tubulin folding cofactors: half a dozen for a dimer. Curr Biol. 2002;12:767–769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

