Creating a novel origin of replication through modulating DNA-protein interfaces
- PMID: 20107513
- PMCID: PMC2809752
- DOI: 10.1371/journal.pone.0008850
Creating a novel origin of replication through modulating DNA-protein interfaces
Abstract
Background: While the molecular mechanisms of DNA-protein specificity at the origin of replication have been determined in many model organisms, these interactions remain unknown in the majority of higher eukaryotes and numerous vertebrate viruses. Similar to many viral origins of replication, adeno-associated virus (AAV) utilizes a cis-acting origin of replication and a virus specific Replication protein (Rep) to faithfully carry out self-priming replication. The mechanisms of AAV DNA replication are generally well understood. However, the molecular basis of specificity between the Rep protein and the viral origin of replication between different AAV serotypes remains uncharacterized.
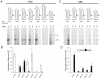
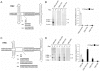
Methodology/principal findings: By generating a panel of chimeric and mutant origins between two AAV serotypes, we have mapped two independent DNA-Protein interfaces involved in replicative specificity. In vivo replication assays and structural modeling demonstrated that three residues in the AAV2 Rep active site are necessary to cleave its cognate origin. An analogous origin (AAV5) possesses a unique interaction between an extended Rep binding element and a 49 aa region of Rep containing two DNA binding interfaces.
Conclusions/significance: The elucidation of these structure-function relationships at the AAV origin led to the creation of a unique recombinant origin and compatible Rep protein with properties independent of either parent serotype. This novel origin may impact the safety and efficacy of AAV as a gene delivery tool. This work may also explain the unique ability of certain AAV serotypes to achieve site-directed integration into the human chromosome. Finally, this result impacts the study of conserved DNA viruses which employ rolling circle mechanisms of replication.
Conflict of interest statement
Figures





References
-
- Im DS, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. - PubMed
-
- Hauswirth WW, Berns KI. Origin and termination of adeno-associated virus DNA replication. Virology. 1977;78:488–499. - PubMed
-
- Hermonat PL, Batchu RB. The adeno-associated virus Rep78 major regulatory protein forms multimeric complexes and the domain for this activity is contained within the carboxy-half of the molecule. FEBS Lett. 1997;20:180–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

