Differential localization and independent acquisition of the H3K9me2 and H3K9me3 chromatin modifications in the Caenorhabditis elegans adult germ line
- PMID: 20107519
- PMCID: PMC2809760
- DOI: 10.1371/journal.pgen.1000830
Differential localization and independent acquisition of the H3K9me2 and H3K9me3 chromatin modifications in the Caenorhabditis elegans adult germ line
Abstract
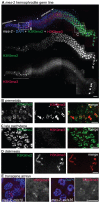
Histone methylation is a prominent feature of eukaryotic chromatin that modulates multiple aspects of chromosome function. Methyl modification can occur on several different amino acid residues and in distinct mono-, di-, and tri-methyl states. However, the interplay among these distinct modification states is not well understood. Here we investigate the relationships between dimethyl and trimethyl modifications on lysine 9 of histone H3 (H3K9me2 and H3K9me3) in the adult Caenorhabditis elegans germ line. Simultaneous immunofluorescence reveals very different temporal/spatial localization patterns for H3K9me2 and H3K9me3. While H3K9me2 is enriched on unpaired sex chromosomes and undergoes dynamic changes as germ cells progress through meiotic prophase, we demonstrate here that H3K9me3 is not enriched on unpaired sex chromosomes and localizes to all chromosomes in all germ cells in adult hermaphrodites and until the primary spermatocyte stage in males. Moreover, high-copy transgene arrays carrying somatic-cell specific promoters are highly enriched for H3K9me3 (but not H3K9me2) and correlate with DAPI-faint chromatin domains. We further demonstrate that the H3K9me2 and H3K9me3 marks are acquired independently. MET-2, a member of the SETDB histone methyltransferase (HMTase) family, is required for all detectable germline H3K9me2 but is dispensable for H3K9me3 in adult germ cells. Conversely, we show that the HMTase MES-2, an E(z) homolog responsible for H3K27 methylation in adult germ cells, is required for much of the germline H3K9me3 but is dispensable for H3K9me2. Phenotypic analysis of met-2 mutants indicates that MET-2 is nonessential for fertility but inhibits ectopic germ cell proliferation and contributes to the fidelity of chromosome inheritance. Our demonstration of the differential localization and independent acquisition of H3K9me2 and H3K9me3 implies that the trimethyl modification of H3K9 is not built upon the dimethyl modification in this context. Further, these and other data support a model in which these two modifications function independently in adult C. elegans germ cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
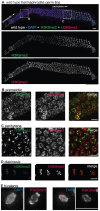

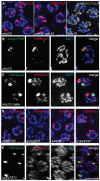

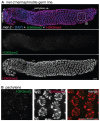
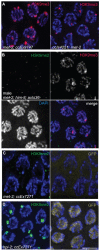

References
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. - PubMed
-
- Yasuhara JC, Wakimoto BT. Molecular landscape of modified histones in Drosophila heterochromatic genes and euchromatin-heterochromatin transition zones. PLoS Genet. 2008;4:e16. doi: 10.1371/journal.pgen.0040016. - DOI - PMC - PubMed
-
- Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. - PubMed
-
- Grewal SI, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8:35–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

