Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines
- PMID: 20107521
- PMCID: PMC2811377
- DOI: 10.3390/v1030920
Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines
Abstract
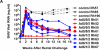
Groups of rhesus macaques that had previously been immunized with HIV-1 envelope (env) peptides and first generation adenovirus serotype 5 (FG-Ad5) vaccines expressing the same peptides were immunized intramuscularly three times with helper-dependent adenovirus (HD-Ad) vaccines expressing only the HIV-1 envelope from JRFL. No gag, pol, or other SHIV genes were used for vaccination. One group of the FG-Ad5-immune animals was immunized three times with HD-Ad5 expressing env. One group was immunized by serotype-switching with HD-Ad6, HD-Ad1, and HD-Ad2 expressing env. Previous work demonstrated that serum antibody levels against env were significantly higher in the serotype-switched group than in the HD-Ad5 group. In this study, neutralizing antibody and T cell responses were compared between the groups before and after rectal challenge with CCR5-tropic SHIV-SF162P3. When serum samples were assayed for neutralizing antibodies, only weak activity was observed. T cell responses against env epitopes were higher in the serotype-switched group. When these animals were challenged rectally with SHIV-SF162P3, both the Ad5 and serotype-switch groups significantly reduced peak viral loads 2 to 10-fold 2 weeks after infection. Peak viral loads were significantly lower for the serotype-switched group as compared to the HD-Ad5-immunized group. Viral loads declined over 18 weeks after infection with some animals viremia reducing nearly 4 logs from the peak. These data demonstrate significant mucosal vaccine effects after immunization with only env antigens. These data also demonstrate HD-Ad vectors are a robust platform for vaccination.
Figures




References
-
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion of the nef gene. Science. 1992;258:1938–1941. - PubMed
-
- Igarashi T, Ami Y, Yamamoto H, Shibata R, Kuwata T, Mukai R, Shinohara K, Komatsu T, Adachi A, Hayami M. Protection of monkeys vaccinated with vpr- and/or nef-defective simian immunodeficiency virus strain mac/human immunodeficiency virus type 1 chimeric viruses: a potential candidate live-attenuated human AIDS vaccine. J Gen Virol. 1997;78:985–989. - PubMed
-
- Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo RC, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. - PMC - PubMed
-
- Ourmanov I, Brown CR, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch VM. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. - PMC - PubMed
-
- Mossman SP, Bex F, Berglund P, Arthos J, O'Neil SP, Riley D, Maul DH, Bruck C, Momin P, Burny A, Fultz PN, Mullins JI, Liljestrom P, Hoover EA. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953–1960. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
