Heme oxygenase-1: its therapeutic roles in inflammatory diseases
- PMID: 20107533
- PMCID: PMC2803295
- DOI: 10.4110/in.2009.9.1.12
Heme oxygenase-1: its therapeutic roles in inflammatory diseases
Abstract
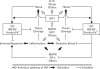
Heme oxygenase (HO)-1 is an inducible enzyme that catalyzes the first and rate-limiting step in the oxidative degradation of free heme into ferrous iron, carbon monoxide (CO), and biliverdin (BV), the latter being subsequently converted into bilirubin (BR). HO-1, once expressed during inflammation, forms high concentrations of its enzymatic by-products that can influence various biological events, and this expression is proven to be associated with the resolution of inflammation. The degradation of heme by HO-1 itself, the signaling actions of CO, the antioxidant properties of BV/BR, and the sequestration of ferrous iron by ferritin all concertedly contribute to the anti-inflammatory effects of HO-1. This review focuses on the anti-inflammatory mechanisms of HO-1 actions and its roles in inflammatory diseases.
Keywords: bilirubin/biliverdin; carbon monoxide; heme oxygenase-1; inflammation; mitogen-activated protein kinase; nuclear factor E2-related factor-2.
Conflict of interest statement
Disclosures: The authors have no financial conflict of interest.
Figures
References
-
- Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. - PubMed
-
- Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. - PubMed
-
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. - PubMed
-
- Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551–571. - PubMed
-
- Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. - PubMed
LinkOut - more resources
Full Text Sources

