Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes
- PMID: 20107601
- PMCID: PMC2809766
- DOI: 10.1371/journal.ppat.1000732
Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes
Abstract
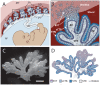
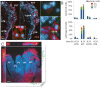
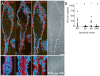
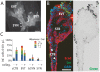
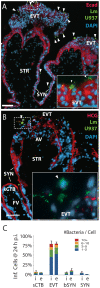
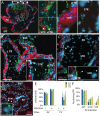
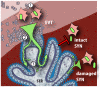
Listeria monocytogenes is an important cause of maternal-fetal infections and serves as a model organism to study these important but poorly understood events. L. monocytogenes can infect non-phagocytic cells by two means: direct invasion and cell-to-cell spread. The relative contribution of each method to placental infection is controversial, as is the anatomical site of invasion. Here, we report for the first time the use of first trimester placental organ cultures to quantitatively analyze L. monocytogenes infection of the human placenta. Contrary to previous reports, we found that the syncytiotrophoblast, which constitutes most of the placental surface and is bathed in maternal blood, was highly resistant to L. monocytogenes infection by either internalin-mediated invasion or cell-to-cell spread. Instead, extravillous cytotrophoblasts-which anchor the placenta in the decidua (uterine lining) and abundantly express E-cadherin-served as the primary portal of entry for L. monocytogenes from both extracellular and intracellular compartments. Subsequent bacterial dissemination to the villous stroma, where fetal capillaries are found, was hampered by further cellular and histological barriers. Our study suggests the placenta has evolved multiple mechanisms to resist pathogen infection, especially from maternal blood. These findings provide a novel explanation why almost all placental pathogens have intracellular life cycles: they may need maternal cells to reach the decidua and infect the placenta.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. - PubMed
-
- Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. - PubMed
-
- Medawar PB. 1953. pp. 320–338. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates.
-
- Drevets DA, Bronze MS. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol 2008 - PubMed
-
- Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9:1236–1243. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

