Polyoma virus-induced osteosarcomas in inbred strains of mice: host determinants of metastasis
- PMID: 20107604
- PMCID: PMC2809769
- DOI: 10.1371/journal.ppat.1000733
Polyoma virus-induced osteosarcomas in inbred strains of mice: host determinants of metastasis
Abstract
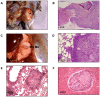
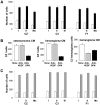
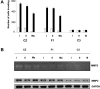
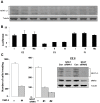
The mouse polyoma virus induces a broad array of solid tumors in mice of many inbred strains. In most strains tumors grow rapidly but fail to metastasize. An exception has been found in the Czech-II/Ei mouse in which bone tumors metastasize regularly to the lung. These tumors resemble human osteosarcoma in their propensity for pulmonary metastasis. Cell lines established from these metastatic tumors have been compared with ones from non-metastatic osteosarcomas arising in C3H/BiDa mice. Osteopontin, a chemokine implicated in migration and metastasis, is known to be transcriptionally induced by the viral middle T antigen. Czech-II/Ei and C3H/BiDa tumor cells expressed middle T and secreted osteopontin at comparable levels as the major chemoattractant. The tumor cell lines migrated equally well in response to recombinant osteopontin as the sole attractant. An important difference emerged in assays for invasion in which tumor cells from Czech-II/Ei mice were able to invade across an extracellular matrix barrier while those from C3H/BiDa mice were unable to invade. Invasive behavior was linked to elevated levels of the metalloproteinase MMP-2 and of the transcription factor NFAT. Inhibition of either MMP-2 or NFAT inhibited invasion by Czech-II/Ei osteosarcoma cells. The metastatic phenotype is dominant in F1 mice. Osteosarcoma cell lines from F1 mice expressed intermediate levels of MMP-2 and NFAT and were invasive. Osteosarcomas in Czech-II/Ei mice retain functional p53. This virus-host model of metastasis differs from engineered models targeting p53 or pRb and provides a system for investigating the genetic and molecular basis of bone tumor metastasis in the absence of p53 loss.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Molloy T, van 't Veer LJ. Recent advances in metastasis research. Curr Opin Genet Dev. 2008;18:35–41. - PubMed
-
- Cardiff RD, Munn RJ, Galvez JJ. Chapter 24. The Tumor Pathology of Genetically Engineered Mice: A New Approach to Molecular Pathology. 2007. pp. 581–622. In The Mouse in Biomedical Research, 2nd Edition JJ Fox, MT Davisson, FW Quimby, AW Barthold, CE Newcomer, AL Smith, editors Academic Press:
-
- Bilger A, Shoemaker AR, Gould KA, Dove WF. Manipulation of the mouse germline in the study of Min-induced neoplasia. Semin Cancer Biol. 1996;7:249–260. - PubMed
-
- Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, et al. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. - PubMed
-
- Balmain A, Nagase H. Cancer resistance genes in mice: models for the study of tumour modifiers. Trends Genet. 1998;14:139–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

