Microtubule targeting agents: from biophysics to proteomics
- PMID: 20107862
- PMCID: PMC11115596
- DOI: 10.1007/s00018-009-0245-6
Microtubule targeting agents: from biophysics to proteomics
Abstract
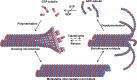
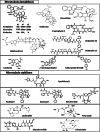
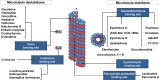
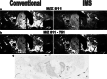
This review explores various aspects of the interaction between microtubule targeting agents and tubulin, including binding site, affinity, and drug resistance. Starting with the basics of tubulin polymerization and microtubule targeting agent binding, we then highlight how the three-dimensional structures of drug-tubulin complexes obtained on stabilized tubulin are seeded by precise biological and biophysical data. New avenues opened by thermodynamics analysis, high throughput screening, and proteomics for the molecular pharmacology of these drugs are presented. The amount of data generated by biophysical, proteomic and cellular techniques shed more light onto the microtubule-tubulin equilibrium and tubulin-drug interaction. Combining these approaches provides new insight into the mechanism of action of known microtubule interacting agents and rapid in-depth characterization of next generation molecules targeting the interaction between microtubules and associated modulators of their dynamics. This will facilitate the design of improved and/or alternative chemotherapies targeting the microtubule cytoskeleton.
Figures
Similar articles
-
The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization.J Mol Biol. 2014 Apr 17;426(8):1848-60. doi: 10.1016/j.jmb.2014.02.005. Epub 2014 Feb 11. J Mol Biol. 2014. PMID: 24530796
-
Structural comparison of the interaction of tubulin with various ligands affecting microtubule dynamics.Curr Cancer Drug Targets. 2012 Jul;12(6):658-66. doi: 10.2174/156800912801784893. Curr Cancer Drug Targets. 2012. PMID: 22385515 Review.
-
Microtubule Targeting Agents as Cancer Chemotherapeutics: An Overview of Molecular Hybrids as Stabilizing and Destabilizing Agents.Curr Top Med Chem. 2017;17(22):2523-2537. doi: 10.2174/1568026617666170104145640. Curr Top Med Chem. 2017. PMID: 28056738 Review.
-
What tubulin drugs tell us about microtubule structure and dynamics.Semin Cell Dev Biol. 2011 Dec;22(9):916-26. doi: 10.1016/j.semcdb.2011.09.014. Epub 2011 Oct 5. Semin Cell Dev Biol. 2011. PMID: 22001382 Review.
-
Novel fragment-derived colchicine-site binders as microtubule-destabilizing agents.Eur J Med Chem. 2022 Nov 5;241:114614. doi: 10.1016/j.ejmech.2022.114614. Epub 2022 Jul 16. Eur J Med Chem. 2022. PMID: 35939994
Cited by
-
Combination of albendazole and 2-methoxyestradiol significantly improves the survival of HCT-116 tumor-bearing nude mice.BMC Cancer. 2013 Feb 23;13:86. doi: 10.1186/1471-2407-13-86. BMC Cancer. 2013. PMID: 23432760 Free PMC article.
-
Thematic Minireview Series: The State of the Cytoskeleton in 2015.J Biol Chem. 2015 Jul 10;290(28):17133-6. doi: 10.1074/jbc.R115.663716. Epub 2015 May 8. J Biol Chem. 2015. PMID: 25957399 Free PMC article. Review.
-
NMK-TD-100, a novel microtubule modulating agent, blocks mitosis and induces apoptosis in HeLa cells by binding to tubulin.PLoS One. 2013 Oct 7;8(10):e76286. doi: 10.1371/journal.pone.0076286. eCollection 2013. PLoS One. 2013. PMID: 24116100 Free PMC article.
-
Support of a free radical mechanism for enhanced antitumor efficacy of the microtubule disruptor OXi4503.Microvasc Res. 2011 Jan;81(1):44-51. doi: 10.1016/j.mvr.2010.10.003. Epub 2010 Oct 23. Microvasc Res. 2011. PMID: 20974154 Free PMC article.
-
Induction of the intrinsic apoptotic pathway via a new antimitotic agent in an esophageal carcinoma cell line.Cell Biosci. 2014 Nov 20;4:68. doi: 10.1186/2045-3701-4-68. eCollection 2014. Cell Biosci. 2014. PMID: 25937890 Free PMC article.
References
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. - PubMed
-
- Nogales E, Wang HW. Structural intermediates in microtubule assembly and disassembly: how and why? Curr Opin Cell Biol. 2006;18:179–184. - PubMed
-
- Ballestrem C, Magid N, Zonis J, Shtutman M, Bershadsky A. Interplay between the actin cytoskeleton, focal adhesions, and microtubules. In: Ridley A, Peckham M, Clark P, editors. Cell motility. From molecules to organisms. Chichester: Wiley; 2004. pp. 75–99.
-
- Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970;24:625–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

