Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling
- PMID: 20108250
- PMCID: PMC3723413
- DOI: 10.1002/jcb.22486
Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling
Abstract
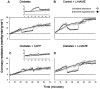
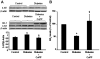
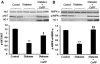
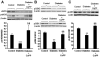
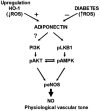
Previous studies related impaired myocardial microcirculation in diabetes to oxidative stress and endothelial dysfunction. Thus, this study was aimed to determine the effect of up-regulating pAMPK-pAKT signaling on coronary microvascular reactivity in the isolated heart of diabetic mice. We measured coronary resistance in wild-type and streptozotocin (STZ)-treated mice, during perfusion pressure changes. Glucose, insulin, and adiponectin levels in plasma and superoxide formation, NOx levels and heme oxygenase (HO) activity in myocardial tissue were determined. In addition, the expression of HO-1, 3-nitrotyrosine, pLKB1, pAMPK, pAKT, and peNOS proteins in control and diabetic hearts were measured. Coronary response to changes in perfusion pressure diverged from control in a time-dependent manner following STZ administration. The responses observed at 28 weeks of diabetes (the maximum time examined) were mimicked by L-NAME administration to control animals and were associated with a decrease in serum adiponectin and myocardial pLKB1, pAMPK, pAKT, and pGSK-3 expression. Cobalt protoporphyrin treatment to induce HO-1 expression reversed the microvascular reactivity seen in diabetes towards that of controls. Up-regulation of HO-1 was associated with an increase in adiponectin, pLKB1, pAKT, pAMPK, pGSK-3, and peNOS levels and a decrease in myocardial superoxide and 3-nitrotyrosine levels. In the present study we describe the time course of microvascular functional changes during the development of diabetes and the existence of a unique relationship between the levels of serum adiponectin, pLKB1, pAKT, and pAMPK activation in diabetic hearts. The restoration of microvascular function suggests a new therapeutic approach to even advanced cardiac microvascular derangement in diabetes.
Copyright 2010 Wiley-Liss, Inc.
Figures


References
-
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. - PubMed
-
- Abraham NG, Kushida T, McClung J, Weiss M, Quan S, Lafaro R, Darzynkiewicz Z, Wolin M. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ Res. 2003;93:507–514. - PubMed
-
- Al-Khalili L, Krook A, Zierath JR, Cartee GD. Prior serum- and AICAR-induced AMPK activation in primary human myocytes does not lead to subsequent increase in insulin-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2004;287:E553–E557. - PubMed
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. - PubMed
-
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

