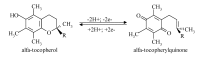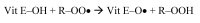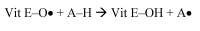So many options but one choice: the human body prefers alpha-tocopherol. A matter of stereochemistry
- PMID: 20108516
- PMCID: PMC5654212
So many options but one choice: the human body prefers alpha-tocopherol. A matter of stereochemistry
Abstract
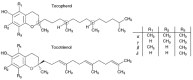
alpha-Tocopherol belongs to the group of vitamin E vitamers. Recent years findings indicate that alpha-tocopherol is more than just a simple fat-soluble anti-oxidant as it was found that it can also regulate gene expression. From all vitamin E vitamers human body preferentially retains alpha-tocopherol, but the reasons for this preference are still elusive. Different studies indicated that human body, through the action of two hepatic proteins, alpha-tocopherol transfer protein (alpha-TTP) and cytochrome P450 4F2 (CYP4F2), is able to make subtle structural differences between different vitamin E forms. This is an example of stereochemistry used as a discrimination factor between molecules with different biological activities.
Figures
Similar articles
-
α-Tocopherol status and altered expression of α-tocopherol-related proteins in streptozotocin-induced type 1 diabetes in rat models.J Nutr Sci Vitaminol (Tokyo). 2014;60(6):380-6. doi: 10.3177/jnsv.60.380. J Nutr Sci Vitaminol (Tokyo). 2014. PMID: 25866300
-
CYP4F2 repression and a modified alpha-tocopherol (vitamin E) metabolism are two independent consequences of ethanol toxicity in human hepatocytes.Toxicol In Vitro. 2017 Apr;40:124-133. doi: 10.1016/j.tiv.2016.12.014. Epub 2017 Jan 3. Toxicol In Vitro. 2017. PMID: 28062356
-
Selective accumulation of alpha-tocopherol in Drosophila is associated with cytochrome P450 tocopherol-omega-hydroxylase activity but not alpha-tocopherol transfer protein.Biochem Biophys Res Commun. 2005 Dec 23;338(3):1537-41. doi: 10.1016/j.bbrc.2005.10.124. Epub 2005 Nov 2. Biochem Biophys Res Commun. 2005. PMID: 16289043
-
A history of vitamin E.Ann Nutr Metab. 2012;61(3):207-12. doi: 10.1159/000343106. Epub 2012 Nov 26. Ann Nutr Metab. 2012. PMID: 23183290 Review.
-
Regulation of xenobiotic metabolism, the only signaling function of alpha-tocopherol?Mol Nutr Food Res. 2010 May;54(5):661-8. doi: 10.1002/mnfr.200900440. Mol Nutr Food Res. 2010. PMID: 20169584 Review.
Cited by
-
Vitamin E Supplementation and Mitochondria in Experimental and Functional Hyperthyroidism: A Mini-Review.Nutrients. 2019 Dec 1;11(12):2900. doi: 10.3390/nu11122900. Nutrients. 2019. PMID: 31805673 Free PMC article. Review.
-
Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway.Biomolecules. 2020 Apr 9;10(4):577. doi: 10.3390/biom10040577. Biomolecules. 2020. PMID: 32283796 Free PMC article.
-
Inclusion of Vitamin E in 1D Nanochannels of 2,4,6-Tris(4-chlorophenoxy)-1,3,5-triazine: Structural and Thermal Characterization of a Stable Solid-State Complex.ACS Omega. 2025 Jun 10;10(24):25819-25828. doi: 10.1021/acsomega.5c02012. eCollection 2025 Jun 24. ACS Omega. 2025. PMID: 40584306 Free PMC article.
-
Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI.PLoS One. 2014 Apr 11;9(4):e94001. doi: 10.1371/journal.pone.0094001. eCollection 2014. PLoS One. 2014. PMID: 24728198 Free PMC article.
-
Alpha-Tocopherol Modulates Transcriptional Activities that Affect Essential Biological Processes in Bovine Cells.Gene Regul Syst Bio. 2010 Nov 22;4:109-24. doi: 10.4137/GRSB.S6007. Gene Regul Syst Bio. 2010. PMID: 21157515 Free PMC article.
References
-
- Cercasov C, Manolescu B. Biochemical and therapeutical aspects of vitamin E. Rev. Roum. Chim. 2005;50(6):419–432.
-
- Weiser H, Vecchi H. Stereoisomers of alpha-tocopheryl acetate. II. Biopotencies of all eight stereoisomers, individually or in mixtures, as determined by rat resorption-gestation tests. Int. J. Vitam. Nutr. Res. 1982;52(3):351–370. - PubMed
-
- Weiser H, Riss G, Kormann AW. Biodiscrimination of the eight α-tocopherol stereoisomers results in preferential accumulation of the four 2R forms in tissues and plasma of rats. J. Nutr. 1996;126:2539–2549. - PubMed
-
- Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. - PubMed
-
- Azzi A, Zingg J-M. Vitamin E: textbooks require updating. Biochem. Mol. Biol. Educat. 2005;33(3):184–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources