Cancer therapies utilizing the camptothecins: a review of the in vivo literature
- PMID: 20108971
- PMCID: PMC3733266
- DOI: 10.1021/mp900243b
Cancer therapies utilizing the camptothecins: a review of the in vivo literature
Abstract
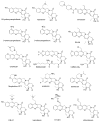
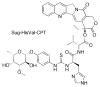
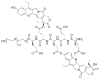
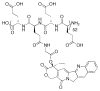
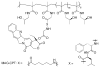
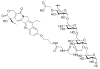
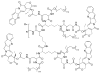
This review summarizes the in vivo assessment-preliminary, preclinical, and clinical-of chemotherapeutics derived from camptothecin or a derivative. Camptothecin is a naturally occurring, pentacyclic quinoline alkaloid that possesses high cytotoxic activity in a variety of cell lines. Major limitations of the drug, including poor solubility and hydrolysis under physiological conditions, prevent full clinical utilization. Camptothecin remains at equilibrium in an active lactone form and inactive hydrolyzed carboxylate form. The active lactone binds to DNA topoisomerase I cleavage complex, believed to be the single site of activity. Binding inhibits DNA religation, resulting in apoptosis. A series of small molecule camptothecin derivatives have been developed that increase solubility, lactone stability and bioavailability to varying levels of success. A number of macromolecular agents have also been described wherein camptothecin(s) are covalently appended or noncovalently associated with the goal of improving solubility and lactone stability, while taking advantage of the tumor physiology to deliver larger doses of drug to the tumor with lower systemic toxicity. With the increasing interest in drug delivery and polymer therapeutics, additional constructs are anticipated. The goal of this review is to summarize the relevant literature for others interested in the field of camptothecin-based therapeutics, specifically in the context of biodistribution, dosing regimens, and pharmacokinetics with the desire of providing a useful source of comparative data. To this end, only constructs where in vivo data is available are reported. The review includes published reports in English through mid-2009.
Figures
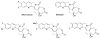
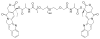
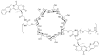
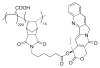

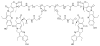
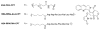

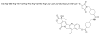
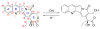
References
-
- Wall ME, Wani MC, Cooke CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88:3888–3890.
-
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agentsVI The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. - PubMed
-
- Slichenmyer WJ, Von Hoff DD. New natural products in cancer chemotherapy. J Clin Pharmacol. 1990;30:770–788. - PubMed
-
- Wall ME. Camptothecin and taxol: discovery to clinic. Med Res Rev. 1998;18:299–314. - PubMed
-
- Hsiang Y-H, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

