Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients
- PMID: 20109122
- PMCID: PMC3123836
- DOI: 10.1089/jamp.2009.0772
Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients
Abstract
Background: When inhaling medication, it is essential that drug particles are delivered to all sites of lung inflammation, including the peripheral airways. The aim of this study was to assess the lung deposition and lung distribution of beclomethasone dipropionate (BDP)/formoterol (100/6 microg), both dissolved in hydrofluoroalkane (HFA) and delivered by pressurized metered dose inhaler (pMDI) in healthy subjects, asthmatic, and chronic obstructive pulmonary disease (COPD) patients, to investigate how the in vitro characteristics of the formulation translate into the in vivo performance in diseases with different airway obstruction.
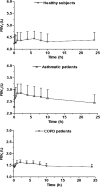
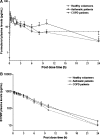
Methods: Healthy volunteers (n = 8), persistent asthmatics (n = 8), and patients with stable COPD (n = 8) completed this open-label, single-dose parallel-group study. Each patient received one single treatment of four puffs of (99 m)Tc-labeled BDP/formoterol formulation. The correlation between particle size distribution of radioactivity and of the drugs in the radiolabeled formulation was validated. Intra- and extrapulmonary deposition, amount of exhaled drug, and the central to peripheral ratio (C/P) were calculated immediately after inhalation. Patients' lung function and pharmacokinetic parameters were also assessed up to 24 h post-dose.
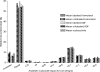
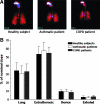
Results: The average lung deposition of BDP/formoterol was 34.08 +/- 9.30% (relative to nominal dose) in healthy subjects, 30.86 +/- 8.89% in asthmatics, and 33.10 +/- 8.90% in COPD patients. Extrathoracic deposition was 53.48% +/- 8.95, 57.64% +/- 9.92 and 54.98% +/- 7.01, respectively. C/P ratios of 1.42 +/- 0.32 in healthy subjects, 1.96 +/- 0.43 in asthmatics, and 1.94 +/- 0.69 for COPD patients confirmed drug distribution to all regions of the lungs. Forced expiratory volume in 1 sec (FEV(1)) increased in all groups after BDP/formoterol inhalation, but was more evident in the patient groups. No significant correlation between baseline lung function and drug deposition was observed. Formoterol, BDP, and beclomethasone 17 monopropionate (B17MP) plasma profiles were comparable between groups.
Conclusion: Inhalation of BDP/formoterol HFA (100/6 microg) produces high and homogeneous deposition of BDP and formoterol in the airways, regardless of pathophysiological condition.
Figures

References
-
- Global Initiative for Asthma (GINA) http://www.GINA.com. http://www.GINA.com
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2007. http://www.goldcopd.org. http://www.goldcopd.org - PubMed
-
- Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182–191. - PubMed
-
- Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med. 1995;332:868–875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
