Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole
- PMID: 20109161
- PMCID: PMC2901525
- DOI: 10.1111/j.1462-5822.2010.01443.x
Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole
Abstract
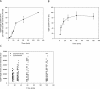
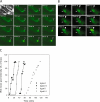
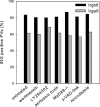
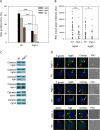
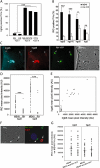
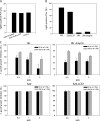
The immunity-related GTPases (IRGs) constitute an interferon-induced intracellular resistance mechanism in mice against Toxoplasma gondii. IRG proteins accumulate on the parasitophorous vacuole membrane (PVM), leading to its disruption and to death of the parasite. How IRGs target the PVM is unknown. We show that accumulation of IRGs on the PVM begins minutes after parasite invasion and increases for about 1 h. Targeting occurs independently of several signalling pathways and the microtubule network, suggesting that IRG transport is diffusion-driven. The intensity of IRG accumulation on the PVM, however, is reduced in absence of the autophagy regulator, Atg5. In wild-type cells IRG proteins accumulate cooperatively on PVMs in a definite order reflecting a temporal hierarchy, with Irgb6 and Irgb10 apparently acting as pioneers. Loading of IRG proteins onto the vacuoles of virulent Toxoplasma strains is attenuated and the two pioneer IRGs are the most affected. The polymorphic rhoptry kinases, ROP16, ROP18 and the catalytically inactive proteins, ROP5A-D, are not individually responsible for this effect. Thus IRG proteins protect mice against avirulent strains of Toxoplasma but fail against virulent strains. The complex cooperative behaviour of IRG proteins in resisting Toxoplasma may hint at undiscovered complexity also in virulence mechanisms.
Figures



Similar articles
-
Toxoplasma gondii virulence in mice is determined by the pseudokinase ROP5B and countered by an IRG-GBP protein interplay.Front Immunol. 2025 Jul 9;16:1593785. doi: 10.3389/fimmu.2025.1593785. eCollection 2025. Front Immunol. 2025. PMID: 40703524 Free PMC article.
-
The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response.PLoS Pathog. 2012;8(6):e1002784. doi: 10.1371/journal.ppat.1002784. Epub 2012 Jun 28. PLoS Pathog. 2012. PMID: 22761577 Free PMC article.
-
Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii.PLoS Biol. 2010 Dec 21;8(12):e1000576. doi: 10.1371/journal.pbio.1000576. PLoS Biol. 2010. PMID: 21203588 Free PMC article.
-
Toxoplasma gondii and the Immunity-Related GTPase (IRG) resistance system in mice: a review.Mem Inst Oswaldo Cruz. 2009 Mar;104(2):234-40. doi: 10.1590/s0074-02762009000200016. Mem Inst Oswaldo Cruz. 2009. PMID: 19430648 Review.
-
The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens.Mamm Genome. 2011 Feb;22(1-2):43-54. doi: 10.1007/s00335-010-9293-3. Epub 2010 Oct 30. Mamm Genome. 2011. PMID: 21052678 Free PMC article. Review.
Cited by
-
Interferon-γ restricts Toxoplasma gondii development in murine skeletal muscle cells via nitric oxide production and immunity-related GTPases.PLoS One. 2012;7(9):e45440. doi: 10.1371/journal.pone.0045440. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024821 Free PMC article.
-
Eimeria falciformis infection of the mouse caecum identifies opposing roles of IFNγ-regulated host pathways for the parasite development.Mucosal Immunol. 2014 Jul;7(4):969-82. doi: 10.1038/mi.2013.115. Epub 2013 Dec 25. Mucosal Immunol. 2014. PMID: 24368565
-
A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-γ-Activated Human Cells.mBio. 2015 Sep 8;6(5):e01157-15. doi: 10.1128/mBio.01157-15. mBio. 2015. PMID: 26350966 Free PMC article.
-
Guanylate-binding protein 1 (GBP1) contributes to the immunity of human mesenchymal stromal cells against Toxoplasma gondii.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):1365-1370. doi: 10.1073/pnas.1619665114. Epub 2017 Jan 25. Proc Natl Acad Sci U S A. 2017. PMID: 28123064 Free PMC article.
-
Toxoplasma gondii superinfection and virulence during secondary infection correlate with the exact ROP5/ROP18 allelic combination.mBio. 2015 Feb 24;6(2):e02280. doi: 10.1128/mBio.02280-14. mBio. 2015. PMID: 25714710 Free PMC article.
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Bastin P, Bagherzadeh Z, Matthews KR, Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol Biochem Parasitol. 1996;77:235–239. - PubMed
-
- Boehm U. Studien zur Charakterisierung der zellulaeren Antwort auf Interferon-γ. Cologne: University of Cologne; 1999. p. 193.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

