Rapid determination of tricarboxylic acid cycle enzyme activities in biological samples
- PMID: 20109171
- PMCID: PMC2823639
- DOI: 10.1186/1471-2091-11-5
Rapid determination of tricarboxylic acid cycle enzyme activities in biological samples
Abstract
Background: In the last ten years, deficiencies in tricarboxylic acid cycle (TCAC) enzymes have been shown to cause a wide spectrum of human diseases, including malignancies and neurological and cardiac diseases. A prerequisite to the identification of disease-causing TCAC enzyme deficiencies is the availability of effective enzyme assays.
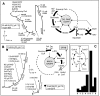
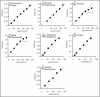
Results: We developed three assays that measure the full set of TCAC enzymes. One assay relies on the sequential addition of reagents to measure succinyl-CoA ligase activity, followed by succinate dehydrogenase, fumarase and, finally, malate dehydrogenase. Another assay measures the activity of alpha-ketoglutarate dehydrogenase followed by aconitase and isocitrate dehydrogenase. The remaining assay measures citrate synthase activity using a standard procedure. We used these assays successfully on extracts of small numbers of human cells displaying various severe or partial TCAC deficiencies and on frozen heart homogenates from heterozygous mice harboring an SDHB gene deletion.
Conclusion: This set of assays is rapid and simple to use and can immediately detect even partial defects, as the activity of each enzyme can be readily compared with one or more other activities measured in the same sample.
Figures

Similar articles
-
Biochemical assays for mitochondrial activity: assays of TCA cycle enzymes and PDHc.Methods Cell Biol. 2007;80:199-222. doi: 10.1016/S0091-679X(06)80010-5. Methods Cell Biol. 2007. PMID: 17445696 Review. No abstract available.
-
Enzymes of the tricarboxylic acid cycle in Ancylostoma ceylanicum and Nippostrongylus brasiliensis.J Parasitol. 1992 Feb;78(1):24-9. J Parasitol. 1992. PMID: 1738065
-
Activities of enzymes of the citric acid cycle and electron transport chain in the skeletal muscle of normal and dystrophic mice (strain 129).Enzyme. 1972;13(5-6):286-92. doi: 10.1159/000459676. Enzyme. 1972. PMID: 4376082 No abstract available.
-
Tricarboxylic acid cycle enzymes of the ectomycorrhizal basidiomycete, Suillus bovinus.Z Naturforsch C J Biosci. 2001 May-Jun;56(5-6):334-42. doi: 10.1515/znc-2001-5-603. Z Naturforsch C J Biosci. 2001. PMID: 11421446
-
Unity and diversity in some bacterial citric acid-cycle enzymes.Adv Microb Physiol. 1981;22:185-244. doi: 10.1016/s0065-2911(08)60328-8. Adv Microb Physiol. 1981. PMID: 7036695 Review. No abstract available.
Cited by
-
Bovine and murine models highlight novel roles for SLC25A46 in mitochondrial dynamics and metabolism, with implications for human and animal health.PLoS Genet. 2017 Apr 4;13(4):e1006597. doi: 10.1371/journal.pgen.1006597. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28376083 Free PMC article.
-
S-glutathionylation reactions in mitochondrial function and disease.Front Cell Dev Biol. 2014 Nov 17;2:68. doi: 10.3389/fcell.2014.00068. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25453035 Free PMC article. Review.
-
ACO2 mutations: A novel phenotype associating severe optic atrophy and spastic paraplegia.Neurol Genet. 2018 Mar 20;4(2):e225. doi: 10.1212/NXG.0000000000000225. eCollection 2018 Apr. Neurol Genet. 2018. PMID: 29564393 Free PMC article. No abstract available.
-
Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells.J Biol Chem. 2013 Dec 20;288(51):36662-75. doi: 10.1074/jbc.M113.466490. Epub 2013 Oct 31. J Biol Chem. 2013. PMID: 24178296 Free PMC article.
-
Mitochondrial CaMKII causes adverse metabolic reprogramming and dilated cardiomyopathy.Nat Commun. 2020 Sep 4;11(1):4416. doi: 10.1038/s41467-020-18165-6. Nat Commun. 2020. PMID: 32887881 Free PMC article.
References
-
- Rustin P, Bourgeron T, Parfait B, Chretien D, Munnich A, Rotig A. Inborn errors of the Krebs cycle: a group of unusual mitochondrial diseases in human. Biochim Biophys Acta. 1997;1361(2):185–197. - PubMed
-
- Tyler D. The Mitochondrion in Health and Diseases. New York: VCH Publishers, Inc; 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

