Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease
- PMID: 20109177
- PMCID: PMC2875645
- DOI: 10.1186/ar2918
Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease
Abstract
Introduction: Fibromyalgia is a chronic pain syndrome with unknown etiology. Recent studies have shown some evidence demonstrating that oxidative stress may have a role in the pathophysiology of fibromyalgia. However, it is still not clear whether oxidative stress is the cause or the effect of the abnormalities documented in fibromyalgia. Furthermore, the role of mitochondria in the redox imbalance reported in fibromyalgia also is controversial. We undertook this study to investigate the role of mitochondrial dysfunction, oxidative stress, and mitophagy in fibromyalgia.
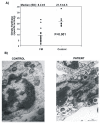
Methods: We studied 20 patients (2 male, 18 female patients) from the database of the Sevillian Fibromyalgia Association and 10 healthy controls. We evaluated mitochondrial function in blood mononuclear cells from fibromyalgia patients measuring, coenzyme Q10 levels with high-performance liquid chromatography (HPLC), and mitochondrial membrane potential with flow cytometry. Oxidative stress was determined by measuring mitochondrial superoxide production with MitoSOX and lipid peroxidation in blood mononuclear cells and plasma from fibromyalgia patients. Autophagy activation was evaluated by quantifying the fluorescence intensity of LysoTracker Red staining of blood mononuclear cells. Mitophagy was confirmed by measuring citrate synthase activity and electron microscopy examination of blood mononuclear cells.
Results: We found reduced levels of coenzyme Q10, decreased mitochondrial membrane potential, increased levels of mitochondrial superoxide in blood mononuclear cells, and increased levels of lipid peroxidation in both blood mononuclear cells and plasma from fibromyalgia patients. Mitochondrial dysfunction was also associated with increased expression of autophagic genes and the elimination of dysfunctional mitochondria with mitophagy.
Conclusions: These findings may support the role of oxidative stress and mitophagy in the pathophysiology of fibromyalgia.
Figures


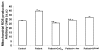


Similar articles
-
Oxidative stress and mitochondrial dysfunction in fibromyalgia.Neuro Endocrinol Lett. 2010;31(2):169-73. Neuro Endocrinol Lett. 2010. PMID: 20424583 Review.
-
Mitochondrial dysfunction in skin biopsies and blood mononuclear cells from two cases of fibromyalgia patients.Clin Biochem. 2010 Sep;43(13-14):1174-6. doi: 10.1016/j.clinbiochem.2010.06.013. Epub 2010 Jul 1. Clin Biochem. 2010. PMID: 20599870
-
Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia?Antioxid Redox Signal. 2013 Nov 20;19(15):1855-60. doi: 10.1089/ars.2013.5346. Epub 2013 May 29. Antioxid Redox Signal. 2013. PMID: 23600892
-
Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient.Nutrition. 2012 Nov-Dec;28(11-12):1200-3. doi: 10.1016/j.nut.2012.03.018. Epub 2012 Aug 14. Nutrition. 2012. PMID: 22898267
-
[Mitochondrial dysfunction in fibromyalgia and its implication in the pathogenesis of disease].Med Clin (Barc). 2011 Mar 12;136(6):252-6. doi: 10.1016/j.medcli.2010.01.030. Epub 2010 Apr 24. Med Clin (Barc). 2011. PMID: 20417529 Review. Spanish.
Cited by
-
Is Disrupted Mitophagy a Central Player to Parkinson's Disease Pathology?Cureus. 2023 Feb 25;15(2):e35458. doi: 10.7759/cureus.35458. eCollection 2023 Feb. Cureus. 2023. PMID: 36860818 Free PMC article. Review.
-
Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin.Cells. 2021 Dec 24;11(1):48. doi: 10.3390/cells11010048. Cells. 2021. PMID: 35011610 Free PMC article.
-
Nutrient Therapy for the Improvement of Fatigue Symptoms.Nutrients. 2023 Apr 30;15(9):2154. doi: 10.3390/nu15092154. Nutrients. 2023. PMID: 37432282 Free PMC article. Review.
-
Study protocol for FIBROKIT: a new tool for fibromyalgia diagnosis and patient follow-up.Front Neurol. 2023 Nov 21;14:1286539. doi: 10.3389/fneur.2023.1286539. eCollection 2023. Front Neurol. 2023. PMID: 38073622 Free PMC article.
-
The Use of the Coenzyme Q10 as a Food Supplement in the Management of Fibromyalgia: A Critical Review.Antioxidants (Basel). 2022 Sep 30;11(10):1969. doi: 10.3390/antiox11101969. Antioxidants (Basel). 2022. PMID: 36290691 Free PMC article. Review.
References
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. - PubMed
-
- Ozgocmen S, Ozyurt H, Sogut S, Akyol O. Current concepts in the pathophysiology of fibromyalgia: the potential role of oxidative stress and nitric oxide. Rheumatol Int. 2006;26:585–597. - PubMed
-
- Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. - PubMed
-
- Cordero MD, Moreno-Fernandez AM, deMiguel M, Bonal P, Campa F, Jimenez-Jimenez LM, Ruiz-Losada A, Sanchez-Dominguez B, Sanchez Alcazar JA, Salviati L, Navas P. Coenzyme Q10 distribution in blood is altered in patients with fibromyalgia. Clin Biochem. 2009;42:732–735. - PubMed

