Acute paretic syndrome in juvenile White Leghorn chickens resembles late stages of acute inflammatory demyelinating polyneuropathies in humans
- PMID: 20109187
- PMCID: PMC2825213
- DOI: 10.1186/1742-2094-7-7
Acute paretic syndrome in juvenile White Leghorn chickens resembles late stages of acute inflammatory demyelinating polyneuropathies in humans
Abstract
Background: Sudden limb paresis is a common problem in White Leghorn flocks, affecting about 1% of the chicken population before achievement of sexual maturity. Previously, a similar clinical syndrome has been reported as being caused by inflammatory demyelination of peripheral nerve fibres. Here, we investigated in detail the immunopathology of this paretic syndrome and its possible resemblance to human neuropathies.
Methods: Neurologically affected chickens and control animals from one single flock underwent clinical and neuropathological examination. Peripheral nervous system (PNS) alterations were characterised using standard morphological techniques, including nerve fibre teasing and transmission electron microscopy. Infiltrating cells were phenotyped immunohistologically and quantified by flow cytometry. The cytokine expression pattern was assessed by quantitative real-time PCR (qRT-PCR). These investigations were accomplished by MHC genotyping and a PCR screen for Marek's disease virus (MDV).
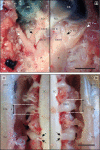
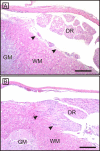
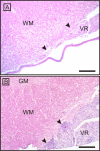
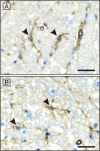
Results: Spontaneous paresis of White Leghorns is caused by cell-mediated, inflammatory demyelination affecting multiple cranial and spinal nerves and nerve roots with a proximodistal tapering. Clinical manifestation coincides with the employment of humoral immune mechanisms, enrolling plasma cell recruitment, deposition of myelin-bound IgG and antibody-dependent macrophageal myelin-stripping. Disease development was significantly linked to a 539 bp microsatellite in MHC locus LEI0258. An aetiological role for MDV was excluded.
Conclusions: The paretic phase of avian inflammatory demyelinating polyradiculoneuritis immunobiologically resembles the late-acute disease stages of human acute inflammatory demyelinating polyneuropathy, and is characterised by a Th1-to-Th2 shift.
Figures






Similar articles
-
Marek's disease as a model for the Landry--Guillain--Barré syndrome: latent viral infection in nonneuronal cells accompanied by specific immune responses to peripheral nerve and myelin.Am J Pathol. 1981 May;103(2):309-20. Am J Pathol. 1981. PMID: 7234967 Free PMC article.
-
A new animal model of spontaneous autoimmune peripheral polyneuropathy: implications for Guillain-Barré syndrome.Acta Neuropathol Commun. 2014 Jan 8;2:5. doi: 10.1186/2051-5960-2-5. Acta Neuropathol Commun. 2014. PMID: 24401681 Free PMC article.
-
The pathogenic relevance of αM-integrin in Guillain-Barré syndrome.Acta Neuropathol. 2016 Nov;132(5):739-752. doi: 10.1007/s00401-016-1599-0. Epub 2016 Jul 26. Acta Neuropathol. 2016. PMID: 27460017 Free PMC article.
-
Guillain-Barré syndrome in northern China. The spectrum of neuropathological changes in clinically defined cases.Brain. 1995 Jun;118 ( Pt 3):577-95. doi: 10.1093/brain/118.3.577. Brain. 1995. PMID: 7600080 Review.
-
Proximal nerve lesions in early Guillain-Barré syndrome: implications for pathogenesis and disease classification.J Neurol. 2017 Feb;264(2):221-236. doi: 10.1007/s00415-016-8204-2. Epub 2016 Jun 17. J Neurol. 2017. PMID: 27314967 Review.
Cited by
-
Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: an update.Biomed Res Int. 2013;2013:852195. doi: 10.1155/2013/852195. Epub 2013 Aug 13. Biomed Res Int. 2013. PMID: 24000328 Free PMC article. Review.
-
Diversity and evolution of the highly polymorphic tandem repeat LEI0258 in the chicken MHC-B region.Immunogenetics. 2013 Jun;65(6):447-59. doi: 10.1007/s00251-013-0697-6. Epub 2013 Mar 26. Immunogenetics. 2013. PMID: 23529664
-
A high-density SNP panel reveals extensive diversity, frequent recombination and multiple recombination hotspots within the chicken major histocompatibility complex B region between BG2 and CD1A1.Genet Sel Evol. 2016 Jan 7;48:1. doi: 10.1186/s12711-015-0181-x. Genet Sel Evol. 2016. PMID: 26743767 Free PMC article.
-
Production and characterization of avian crypt-villus enteroids and the effect of chemicals.BMC Vet Res. 2020 Jun 5;16(1):179. doi: 10.1186/s12917-020-02397-1. BMC Vet Res. 2020. PMID: 32503669 Free PMC article.
-
Immunopathology and Th1/Th2 immune response of Campylobacter jejuni-induced paralysis resembling Guillain-Barré syndrome in chicken.Med Microbiol Immunol. 2012 May;201(2):177-87. doi: 10.1007/s00430-011-0220-3. Epub 2011 Nov 19. Med Microbiol Immunol. 2012. PMID: 22102098
References
-
- Latov N. Diagnosis of CIDP. Neurology. 2002;59:S2–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

