Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells
- PMID: 20109207
- PMCID: PMC2827377
- DOI: 10.1186/1471-2121-11-11
Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells
Abstract
Background: Human multipotent mesenchymal stromal cells (MSC) can be isolated from various tissues including bone marrow. Here, MSC participate as bone lining cells in the formation of the hematopoietic stem cell niche. In this compartment, the oxygen tension is low and oxygen partial pressure is estimated to range from 1% to 7%. We analyzed the effect of low oxygen tensions on human MSC cultured with platelet-lysate supplemented media and assessed proliferation, morphology, chromosomal stability, immunophenotype and plasticity.
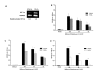
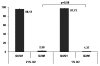
Results: After transferring MSC from atmospheric oxygen levels of 21% to 1%, HIF-1alpha expression was induced, indicating efficient oxygen reduction. Simultaneously, MSC exhibited a significantly different morphology with shorter extensions and broader cell bodies. MSC did not proliferate as rapidly as under 21% oxygen and accumulated in G1 phase. The immunophenotype, however, was unaffected. Hypoxic stress as well as free oxygen radicals may affect chromosomal stability. However, no chromosomal abnormalities in human MSC under either culture condition were detected using high-resolution matrix-based comparative genomic hybridization. Reduced oxygen tension severely impaired adipogenic and osteogenic differentiation of human MSC. Elevation of oxygen from 1% to 3% restored osteogenic differentiation.
Conclusion: Physiologic oxygen tension during in vitro culture of human MSC slows down cell cycle progression and differentiation. Under physiological conditions this may keep a proportion of MSC in a resting state. Further studies are needed to analyze these aspects of MSC in tissue regeneration.
Figures


Similar articles
-
Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability.Cell Physiol Biochem. 2017;44(4):1295-1310. doi: 10.1159/000485490. Epub 2017 Nov 29. Cell Physiol Biochem. 2017. PMID: 29183009
-
Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status.BMC Cell Biol. 2011 Mar 30;12:12. doi: 10.1186/1471-2121-12-12. BMC Cell Biol. 2011. PMID: 21450070 Free PMC article.
-
Modulation of the in vitro angiogenic potential of human mesenchymal stromal cells from different tissue sources.J Cell Physiol. 2020 Oct;235(10):7224-7238. doi: 10.1002/jcp.29622. Epub 2020 Feb 9. J Cell Physiol. 2020. PMID: 32037550
-
[Mesenchymal stromal progenitor cells: general characteristics and functional state in low oxygen tension].Ross Fiziol Zh Im I M Sechenova. 2008 Jul;94(7):737-57. Ross Fiziol Zh Im I M Sechenova. 2008. PMID: 18767387 Review. Russian.
-
Bone marrow mesenchymal stem cells: historical overview and concepts.Hum Gene Ther. 2010 Sep;21(9):1045-56. doi: 10.1089/hum.2010.115. Hum Gene Ther. 2010. PMID: 20565251 Free PMC article. Review.
Cited by
-
Stem cell-based tissue engineering approaches for musculoskeletal regeneration.Curr Pharm Des. 2013;19(19):3429-45. doi: 10.2174/13816128113199990350. Curr Pharm Des. 2013. PMID: 23432679 Free PMC article. Review.
-
Chimeric Self-assembling Nanofiber Containing Bone Marrow Homing Peptide's Motif Induces Motor Neuron Recovery in Animal Model of Chronic Spinal Cord Injury; an In Vitro and In Vivo Investigation.Mol Neurobiol. 2016 Jul;53(5):3298-3308. doi: 10.1007/s12035-015-9266-3. Epub 2015 Jun 11. Mol Neurobiol. 2016. PMID: 26063594
-
HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia.Stem Cell Res Ther. 2015 Mar 12;6(1):21. doi: 10.1186/s13287-015-0014-4. Stem Cell Res Ther. 2015. PMID: 25889814 Free PMC article.
-
Hypoxia-mimetic agents inhibit proliferation and alter the morphology of human umbilical cord-derived mesenchymal stem cells.BMC Cell Biol. 2011 Aug 9;12:32. doi: 10.1186/1471-2121-12-32. BMC Cell Biol. 2011. PMID: 21827650 Free PMC article.
-
Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells.Am J Physiol Cell Physiol. 2010 Dec;299(6):C1562-70. doi: 10.1152/ajpcell.00221.2010. Epub 2010 Sep 22. Am J Physiol Cell Physiol. 2010. PMID: 20861473 Free PMC article.
References
-
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. - PubMed
-
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. - DOI - PMC - PubMed
-
- Müller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F, Dominici M, Greil J, Handgretinger R. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

