Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial
- PMID: 20109213
- PMCID: PMC2834642
- DOI: 10.1186/1465-9921-11-10
Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial
Erratum in
- Respir Res. 2010;11:88
Abstract
Background: Acute exacerbations contribute to the morbidity and mortality associated with chronic obstructive pulmonary disease (COPD). This proof-of-concept study evaluates whether intermittent pulsed moxifloxacin treatment could reduce the frequency of these exacerbations.
Methods: Stable patients with COPD were randomized in a double-blind, placebo-controlled trial to receive moxifloxacin 400 mg PO once daily (N = 573) or placebo (N = 584) once a day for 5 days. Treatment was repeated every 8 weeks for a total of six courses. Patients were repeatedly assessed clinically and microbiologically during the 48-week treatment period, and for a further 24 weeks' follow-up.
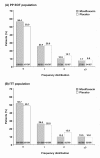
Results: At 48 weeks the odds ratio (OR) for suffering an exacerbation favoured moxifloxacin: per-protocol (PP) population (N = 738, OR 0.75, 95% confidence interval (CI) 0.565-0.994, p = 0.046), intent-to-treat (ITT) population (N = 1149, OR 0.81, 95% CI 0.645-1.008, p = 0.059), and a post-hoc analysis of per-protocol (PP) patients with purulent/mucopurulent sputum production at baseline (N = 323, OR 0.55, 95% CI 0.36-0.84, p = 0.006).There were no significant differences between moxifloxacin and placebo in any pre-specified efficacy subgroup analyses or in hospitalization rates, mortality rates, lung function or changes in St George's Respiratory Questionnaire (SGRQ) total scores. There was, however, a significant difference in favour of moxifloxacin in the SGRQ symptom domain (ITT: -8.2 vs -3.8, p = 0.009; PP: -8.8 vs -4.4, p = 0.006). Moxifloxacin treatment was not associated with consistent changes in moxifloxacin susceptibility. There were more treatment-emergent, drug related adverse events with moxifloxacin vs placebo (p < 0.001) largely due to gastrointestinal events (4.7% vs 0.7%).
Conclusions: Intermittent pulsed therapy with moxifloxacin reduced the odds of exacerbation by 20% in the ITT population, by 25% among the PP population and by 45% in PP patients with purulent/mucopurulent sputum at baseline. There were no unexpected adverse events and there was no evidence of resistance development.
Trial registration: ClinicalTrials.gov number, NCT00473460 (ClincalTrials.gov).
Figures



References
-
- Kanner RE, Anthonisen NR, Connett JE. Lung Health Study Research Group. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:358–364. - PubMed
-
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley PM. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. - DOI - PubMed
-
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

