Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis
- PMID: 20109233
- PMCID: PMC2825214
- DOI: 10.1186/1742-2094-7-8
Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis
Abstract
Background: The role of neuroinflammation in motor neuron death of amyotrophic lateral sclerosis (ALS) is unclear. The human mutant superoxide dismutase-1 (hmSOD1)-expressing murine transgenic model of ALS has provided some insight into changes in microglia activity during disease progression. The purpose of this study was to gain further knowledge by characterizing the immunological changes during disease progression in the spinal cord and peripheral nerve using the more recently developed hmSOD1 rat transgenic model of ALS.
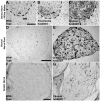
Methods: Using immunohistochemistry, the extent and intensity of tissue CD11b expression in spinal cord, lumbar nerve roots, and sciatic nerve were evaluated in hmSOD1 rats that were pre-clinical, at clinical onset, and near disease end-stage. Changes in CD11b expression were compared to the detection of MHC class II and CD68 microglial activation markers in the ventral horn of the spinal cord, as well as to the changes in astrocytic GFAP expression.
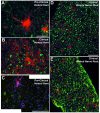
Results: Our study reveals an accumulation of microglia/macrophages both in the spinal cord and peripheral nerve prior to clinical onset based on CD11b tissue expression. The microglia formed focal aggregates in the ventral horn and became more widespread as the disease progressed. Hypertrophic astrocytes were not prominent in the ventral horn until after clinical onset, and the enhancement of GFAP did not have a strong correlation to increased CD11b expression. Detection of MHC class II and CD68 expression was found in the ventral horn only after clinical onset. The macrophages in the ventral nerve root and sciatic nerve of hmSOD1 rats were observed encircling axons.
Conclusions: These findings describe for the first time in the hmSOD1 rat transgenic model of ALS that enhancement of microglia/macrophage activity occurs pre-clinically both in the peripheral nerve and in the spinal cord. CD11b expression is shown to be a superior indicator for early immunological changes compared to other microglia activation markers and astrogliosis. Furthermore, we suggest that the early activity of microglia/macrophages is involved in the early phase of motor neuron degeneration and propose that studies involving immunomodulation in hmSOD1transgenic models need to consider effects on macrophages in peripheral nerves as well as to microglia in the spinal cord.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

