The functional organisation of glia in the adult brain of Drosophila and other insects
- PMID: 20109517
- PMCID: PMC2847375
- DOI: 10.1016/j.pneurobio.2010.01.001
The functional organisation of glia in the adult brain of Drosophila and other insects
Abstract
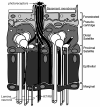
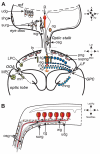
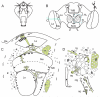
This review annotates and categorises the glia of adult Drosophila and other model insects and analyses the developmental origins of these in the Drosophila optic lobe. The functions of glia in the adult vary depending upon their sub-type and location in the brain. The task of annotating glia is essentially complete only for the glia of the fly's lamina, which comprise: two types of surface glia-the pseudocartridge and fenestrated glia; two types of cortex glia-the distal and proximal satellite glia; and two types of neuropile glia-the epithelial and marginal glia. We advocate that the term subretinal glia, as used to refer to both pseudocartridge and fenestrated glia, be abandoned. Other neuropiles contain similar glial subtypes, but other than the antennal lobes these have not been described in detail. Surface glia form the blood brain barrier, regulating the flow of substances into and out of the nervous system, both for the brain as a whole and the optic neuropiles in particular. Cortex glia provide a second level of barrier, wrapping axon fascicles and isolating neuronal cell bodies both from neighbouring brain regions and from their underlying neuropiles. Neuropile glia can be generated in the adult and a subtype, ensheathing glia, are responsible for cleaning up cellular debris during Wallerian degeneration. Both the neuropile ensheathing and astrocyte-like glia may be involved in clearing neurotransmitters from the extracellular space, thus modifying the levels of histamine, glutamate and possibly dopamine at the synapse to ultimately affect behaviour.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
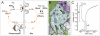
References
-
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

