Aquaporins in spinal cord injury: the janus face of aquaporin 4
- PMID: 20109536
- PMCID: PMC2885549
- DOI: 10.1016/j.neuroscience.2010.01.037
Aquaporins in spinal cord injury: the janus face of aquaporin 4
Abstract
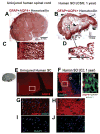
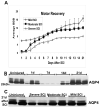
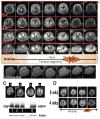
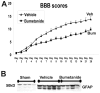
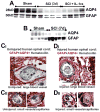
Although malfunction of spinal cord water channels (aquaporins, AQP) likely contributes to severe disturbances in ion/water homeostasis after spinal cord injury (SCI), their roles are still poorly understood. Here we report and discuss the potential significance of changes in the AQP4 expression in human SCI that generates glial fibrillary acidic protein (GFAP)-labeled astrocytes devoid of AQP4, and GFAP-labeled astroglia that overexpress AQP4. We used a rat model of contusion SCI to study observed changes in human SCI. AQP4-negative astrocytes are likely generated during the process of SCI-induced replacement of lost astrocytes, but their origin and role in SCI remains to be investigated. We found that AQP4-overexpression is likely triggered by hypoxia. Our transcriptional profiling of injured rat cords suggests that elevated AQP4-mediated water influx accompanies increased uptake of chloride and potassium ions which represents a protective astrocytic reaction to hypoxia. However, unbalanced water intake also results in astrocytic swelling that can contribute to motor impairment, but likely only in milder injuries. In severe rat SCI, a low abundance of AQP4-overexpressing astrocytes was found during the motor recovery phase. Our results suggest that severe rat contusion SCI is a better model to analyze AQP4 functions after SCI. We found that AQP4 increases in the chronic post-injury phase are associated with the development of pain-like behavior in SCI rats, while possible mechanisms underlying pain development may involve astrocytic swelling-induced glutamate release. In contrast, the formation and size of fluid-filled cavities occurring later after SCI does not appear to be affected by the extent of increased AQP4 levels. Therefore, the effect of therapeutic interventions targeting AQP4 will depend not only on the time interval after SCI or animal models, but also on the balance between protective role of increased AQP4 in hypoxia and deleterious effects of ongoing astrocytic swelling.
Published by Elsevier Ltd.
Figures



References
-
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):2106–11. - PMC - PubMed
-
- Backe HA, Betz RR, Mesgarzadeh M, Beck T, Clancy M. Post-traumatic spinal cord cysts evaluated by magnetic resonance imaging. Paraplegia. 1991;29:607–612. - PubMed
-
- Badaut J. Aquaglyceroporin 9 in brain pathologies. Neuroscience. 2009 - PubMed
-
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005 May;50(3):187–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

