Structure of a human multidrug transporter in an inward-facing conformation
- PMID: 20109555
- PMCID: PMC3232029
- DOI: 10.1016/j.jsb.2010.01.011
Structure of a human multidrug transporter in an inward-facing conformation
Abstract
Multidrug resistance protein 1 (ABCC1) is a member of the 'C' class of ATP-binding cassette transporters, which can give rise to resistance to chemotherapy via drug export from cells. It also acts as a leukotriene C4 transporter, and hence has a role in adaptive immune response. Most C-class members have an additional NH(2)-terminal transmembrane domain versus other ATP-binding cassette transporters, but little is known about the structure and role of this domain. Using electron cryomicroscopy of 2D crystals, data at 1/6per A(-1) resolution was generated for the full-length ABCC1 protein in the absence of ATP. Analysis using homologous structures from bacteria and mammals allowed the core transmembrane domains to be localised in the map. These display an inward-facing conformation and there is a noteworthy separation of the cytoplasmic nucleotide-binding domains. Examination of non-core features in the map suggests that the additional NH(2)-terminal domain has extensive contacts on one side of both core domains, and mirrors their inward-facing configuration in the absence of nucleotide.
Figures
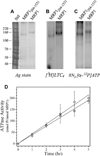

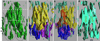


References
-
- Auer M, Scarborough GA, Kuhlbrandt W. Three-dimensional map of the plasma membrane H+-ATPase in the open conformation. Nature. 1998;392:840–843. - PubMed
-
- Auer M, Scarborough GA, Kuhlbrandt W. Surface crystallisation of the plasma membrane H+-ATPase on a carbon support film for electron crystallography. J. Mol. Biol. 1999;287:961–968. - PubMed
-
- Awayn NH, Rosenberg MF, Kamis AB, Aleksandrov LA, Riordan JR, Ford RC. Crystallographic and single-particle analyses of native and nucleotide-bound forms of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Biochem. Soc. Trans. 2005;33:996–999. - PubMed
-
- Bakos E, Hegedus T, Hollo Z, Welker E, Tusnady GE, Zaman GJR, Flens MJ, Varadi A, Sarkadi B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J. Biol. Chem. 1996;271:12322–12326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

