Loss of GATA1 and gain of FLI1 expression during thrombocyte maturation
- PMID: 20110178
- PMCID: PMC2829368
- DOI: 10.1016/j.bcmd.2009.12.012
Loss of GATA1 and gain of FLI1 expression during thrombocyte maturation
Abstract
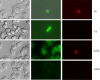
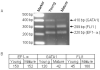
In this paper, we characterized expression of GATA1 and FLI1 gene promoters in thrombocytes of zebrafish transgenic lines, G1-GM2 and TG(fli1:EGFP)y1 that carry transgenes of GATA1 and FLI1 gene promoters driving GFP. We found two discrete populations of thrombocytes verified by morphology, labeled with GFP in both G1-GM2 and TG(fli1:EGFP)y1 lines: (1) the more intensely labeled GFP+ thrombocyte, and (2) the less intensely labeled GFP+ thrombocytes. The more intensely labeled GFP+ thrombocyte in G1-GM2 line and the less intensely labeled GFP+ thrombocytes in the TG(fli1:EGFP)y1 line corresponded to young thrombocytes. These results showed that young thrombocytes have higher GATA1 promoter activity, while mature thrombocytes have more FLI1 gene promoter transcription. This finding suggests that there is a gradual loss of GATA1 and gain of FLI1 expression as the thrombocytes mature, and this overexpression of FLI1 may help maintain the thrombocyte lineage. Furthermore, the presence of transcriptional factors similar to those found in megakaryocytes raises the possibility that vertebrate thrombocytes may be the forerunners of mammalian megakaryocytes and, therefore, could serve as a model to study megakaryocyte maturation.
2009 Elsevier Inc. All rights reserved.
Figures



References
-
- Orkin SH, Shivdasani RA, Fujiwara Y, McDevitt MA. Transcription factor GATA-1 in megakaryocyte development. Stem Cells. 1998;16(Suppl 2):79–83. - PubMed
-
- Cantor AB, Orkin SH. Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins. Semin Cell Dev Biol. 2005;16:117–128. - PubMed
-
- Long Q, Meng A, Wang H, Jessen JR, Farrell MJ, Lin S. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

