Interaction of herpes simplex virus type 2 (HSV-2) glycoprotein D with the host cell surface is sufficient to induce Chlamydia trachomatis persistence
- PMID: 20110302
- PMCID: PMC2889450
- DOI: 10.1099/mic.0.036566-0
Interaction of herpes simplex virus type 2 (HSV-2) glycoprotein D with the host cell surface is sufficient to induce Chlamydia trachomatis persistence
Abstract
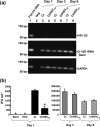
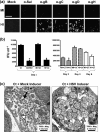
When presented with certain unfavourable environmental conditions, Chlamydia trachomatis reticulate bodies (RBs) enter into a viable, yet non-cultivable state called persistence. Previously, we established an in vitro C. trachomatis and herpes simplex virus type 2 (HSV-2) co-infection model. These data indicate that (i) viral co-infection stimulates chlamydial persistence, (ii) productive HSV replication is not required for persistence induction, and (iii) HSV-induced persistence is not mediated by any currently characterized anti-chlamydial pathway or persistence inducer. In this study we demonstrated that chlamydial infectivity, though initially suppressed, recovered within 44 h of co-infection with UV-inactivated HSV-2, demonstrating that HSV-induced persistence is reversible. Co-incubation of chemically fixed, HSV-2-infected inducer cells with viable, C. trachomatis-infected responder cells both suppressed production of infectious chlamydial progeny and stimulated formation of swollen, aberrantly shaped RBs. In addition, pre-incubation of viral particles with viral glycoprotein D (gD)-specific neutralizing antibody prevented co-infection-induced persistence. Finally, exposure of C. trachomatis-infected cells to a soluble, recombinant HSV-2 gD : Fc fusion protein decreased production of infectious EBs to a degree similar to that observed in co-infected cultures. Thus, we conclude that interaction of HSV gD with the host cell surface is sufficient to trigger a novel host anti-chlamydial response that restricts chlamydial development.
Figures


Similar articles
-
An early event in the herpes simplex virus type-2 replication cycle is sufficient to induce Chlamydia trachomatis persistence.Cell Microbiol. 2007 Mar;9(3):725-37. doi: 10.1111/j.1462-5822.2006.00823.x. Epub 2006 Nov 28. Cell Microbiol. 2007. PMID: 17140408
-
Chlamydia trachomatis enters a viable but non-cultivable (persistent) state within herpes simplex virus type 2 (HSV-2) co-infected host cells.Cell Microbiol. 2006 Jan;8(1):149-62. doi: 10.1111/j.1462-5822.2005.00608.x. Cell Microbiol. 2006. PMID: 16367874
-
Herpes simplex virus co-infection-induced Chlamydia trachomatis persistence is not mediated by any known persistence inducer or anti-chlamydial pathway.Microbiology (Reading). 2008 Mar;154(Pt 3):971-978. doi: 10.1099/mic.0.2007/012161-0. Microbiology (Reading). 2008. PMID: 18310043
-
[Mechanisms of Chlamydia trachomatis and herpes simplex virus persistence during viral-bacterial infection].Zh Mikrobiol Epidemiol Immunobiol. 2009 Sep-Oct;(5):105-10. Zh Mikrobiol Epidemiol Immunobiol. 2009. PMID: 20066779 Review. Russian.
-
Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo.Front Microbiol. 2018 Dec 12;9:3101. doi: 10.3389/fmicb.2018.03101. eCollection 2018. Front Microbiol. 2018. PMID: 30619180 Free PMC article. Review.
Cited by
-
Chlamydial Pre-Infection Protects from Subsequent Herpes Simplex Virus-2 Challenge in a Murine Vaginal Super-Infection Model.PLoS One. 2016 Jan 4;11(1):e0146186. doi: 10.1371/journal.pone.0146186. eCollection 2016. PLoS One. 2016. PMID: 26726882 Free PMC article.
-
Kaposi's sarcoma: a computational approach through protein-protein interaction and gene regulatory networks analysis.Virus Genes. 2013 Apr;46(2):242-54. doi: 10.1007/s11262-012-0865-z. Epub 2012 Dec 25. Virus Genes. 2013. PMID: 23266878
-
Mixed infections with Chlamydia and porcine epidemic diarrhea virus - a new in vitro model of chlamydial persistence.BMC Microbiol. 2010 Jul 27;10:201. doi: 10.1186/1471-2180-10-201. BMC Microbiol. 2010. PMID: 20663197 Free PMC article.
-
Chlamydia persistence -- a tool to dissect chlamydia--host interactions.Microbes Infect. 2011 Jul;13(7):649-62. doi: 10.1016/j.micinf.2011.03.004. Epub 2011 Mar 31. Microbes Infect. 2011. PMID: 21458583 Free PMC article. Review.
-
Ptr/CTL0175 Is Required for the Efficient Recovery of Chlamydia trachomatis From Stress Induced by Gamma-Interferon.Front Microbiol. 2019 Apr 10;10:756. doi: 10.3389/fmicb.2019.00756. eCollection 2019. Front Microbiol. 2019. PMID: 31024512 Free PMC article.
References
-
- Abdelrahman, Y. M. & Belland, R. J. (2005). The chlamydial developmental cycle. FEMS Microbiol Rev 29, 949–959. - PubMed
-
- Amici, C., Rossi, A., Costanzo, A., Ciafre, S., Marinari, B., Balsamo, M., Levrero, M. & Santoro, M. G. (2006). Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J Biol Chem 281, 7110–7117. - PubMed
-
- Bragina, E. Y., Gomberg, M. A. & Dmitriev, G. A. (2001). Electron microscopic evidence of persistent chlamydial infection following treatment. J Eur Acad Dermatol Venereol 15, 405–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

