SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation
- PMID: 20110314
- PMCID: PMC2827686
- DOI: 10.1242/dev.045757
SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation
Abstract
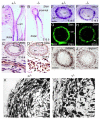
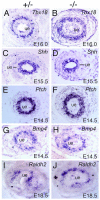
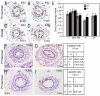
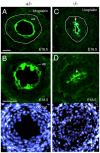
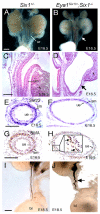
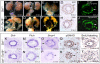
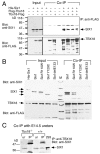
Dysfunction of the ureter often leads to urine flow impairment from the kidney to the bladder, causing dilation of the ureter and/or renal pelvis. Six1 is a crucial regulator of renal development: mutations in human SIX1 cause branchio-oto-renal (BOR) syndrome and Six1(-/-) mice exhibit renal agenesis, although the ureter is present. It remains unclear whether Six1 plays a role in regulating ureter morphogenesis. We demonstrate here that Six1 is differentially expressed during ureter morphogenesis. It was expressed in undifferentiated smooth muscle (SM) progenitors, but was downregulated in differentiating SM cells (SMCs) and had disappeared by E18.5. In Six1(-/-) mice, the ureteral mesenchymal precursors failed to condense and differentiate into normal SMCs and showed increased cell death, indicating that Six1 is required for the maintenance and normal differentiation of SM progenitors. A delay in SMC differentiation was observed in Six1(-/-) ureters. A lack of Six1 in the ureter led to hydroureter and hydronephrosis without anatomical obstruction when kidney formation was rescued in Six1(-/-) embryos by specifically expressing Six1 in the metanephric mesenchyme, but not the ureter, under control of the Eya1 promoter. We show that Six1 and Tbx18 genetically interact to synergistically regulate SMC development and ureter function and that their gene products form a complex in cultured cells and in the developing ureter. Two missense mutations in SIX1 from BOR patients reduced or abolished SIX1-TBX18 complex formation. These findings uncover an essential role for Six1 in establishing a functionally normal ureter and provide new insights into the molecular basis of urinary tract malformations in BOR patients.
Figures


Similar articles
-
Smad4 regulates ureteral smooth muscle cell differentiation during mouse embryogenesis.PLoS One. 2014 Aug 15;9(8):e104503. doi: 10.1371/journal.pone.0104503. eCollection 2014. PLoS One. 2014. PMID: 25127126 Free PMC article.
-
Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis.Dev Biol. 2011 Apr 1;352(1):141-51. doi: 10.1016/j.ydbio.2011.01.027. Epub 2011 Jan 31. Dev Biol. 2011. PMID: 21281623 Free PMC article.
-
SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes.Proc Natl Acad Sci U S A. 2004 May 25;101(21):8090-5. doi: 10.1073/pnas.0308475101. Epub 2004 May 12. Proc Natl Acad Sci U S A. 2004. PMID: 15141091 Free PMC article.
-
Ureter growth and differentiation.Semin Cell Dev Biol. 2014 Dec;36:21-30. doi: 10.1016/j.semcdb.2014.07.014. Epub 2014 Aug 1. Semin Cell Dev Biol. 2014. PMID: 25087982 Review.
-
Branchio-oto-renal syndrome.Am J Med Genet A. 2007 Jul 15;143A(14):1671-8. doi: 10.1002/ajmg.a.31561. Am J Med Genet A. 2007. PMID: 17238186 Review.
Cited by
-
SIX1 gene: absence of mutations in children with isolated congenital anomalies of kidney and urinary tract.J Nephrol. 2014 Dec;27(6):667-71. doi: 10.1007/s40620-014-0112-x. Epub 2014 Jun 5. J Nephrol. 2014. PMID: 24899122
-
Smad4 regulates ureteral smooth muscle cell differentiation during mouse embryogenesis.PLoS One. 2014 Aug 15;9(8):e104503. doi: 10.1371/journal.pone.0104503. eCollection 2014. PLoS One. 2014. PMID: 25127126 Free PMC article.
-
Kruppel-like factor 5 is required for formation and differentiation of the bladder urothelium.Dev Biol. 2011 Oct 1;358(1):79-90. doi: 10.1016/j.ydbio.2011.07.020. Epub 2011 Jul 22. Dev Biol. 2011. PMID: 21803035 Free PMC article.
-
Angiotensin II regulates growth of the developing papillas ex vivo.Am J Physiol Renal Physiol. 2012 May 1;302(9):F1112-20. doi: 10.1152/ajprenal.00435.2011. Epub 2012 Feb 1. Am J Physiol Renal Physiol. 2012. PMID: 22301625 Free PMC article.
-
Six1 transcription factor is critical for coordination of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung.Dev Biol. 2011 May 15;353(2):242-58. doi: 10.1016/j.ydbio.2011.02.031. Epub 2011 Mar 6. Dev Biol. 2011. PMID: 21385574 Free PMC article.
References
-
- Abdelhak S., Kalatzis V., Heilig R., Compain S., Samson D., Vincent C., Weil D., Cruaud C., Sahly I., Leibovici M., et al. (1997). A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat. Genet. 15, 157-164 - PubMed
-
- Brenner-Anantharam A., Cebrian C., Guillaume R., Hurtado R., Sun T. T., Herzlinger D. (2007). Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 134, 1967-1975 - PubMed
-
- Buller C., Xu X., Marquis V., Schwanke R., Xu P. X. (2001). Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 10, 2775-2781 - PubMed
-
- Bush K. T., Vaughn D. A., Li X., Rosenfeld M. G., Rose D. W., Mendoza S. A., Nigam S. K. (2006). Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system. Dev. Biol. 298, 571-584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

