Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2
- PMID: 20110355
- PMCID: PMC2856255
- DOI: 10.1074/jbc.M109.064162
Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2
Abstract
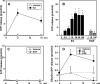
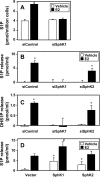
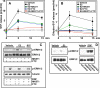
Sphingosine 1-phosphate (S1P), a potent sphingolipid mediator produced by sphingosine kinase isoenzymes (SphK1 and SphK2), regulates diverse cellular processes important for breast cancer progression acting in an autocrine and/or paracrine manner. Here we show that SphK1, but not SphK2, increased S1P export from MCF-7 cells. Whereas for both estradiol (E(2)) and epidermal growth factor-activated SphK1 and production of S1P, only E(2) stimulated rapid release of S1P and dihydro-S1P from MCF-7 cells. E(2)-induced S1P and dihydro-S1P export required estrogen receptor-alpha, not GPR30, and was suppressed either by pharmacological inhibitors or gene silencing of ABCC1 (multidrug resistant protein 1) or ABCG2 (breast cancer resistance protein). Inhibiting these transporters also blocked E(2)-induced activation of ERK1/2, indicating that E(2) activates ERK via downstream signaling of S1P. Taken together, our findings suggest that E(2)-induced export of S1P mediated by ABCC1 and ABCG2 transporters and consequent activation of S1P receptors may contribute to nongenomic signaling of E(2) important for breast cancer pathophysiology.
Figures
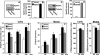




References
-
- Spiegel S., Milstien S. (2003) Nat. Rev. Mol. Cell Biol. 4, 397–407 - PubMed
-
- Hannun Y. A., Obeid L. M. (2008) Nat. Rev. Mol. Cell Biol. 9, 139–150 - PubMed
-
- Saba J. D., Hla T. (2004) Circ. Res. 94, 724–734 - PubMed
-
- Okada T., Ding G., Sonoda H., Kajimoto T., Haga Y., Khosrowbeygi A., Gao S., Miwa N., Jahangeer S., Nakamura S. (2005) J. Biol. Chem. 280, 36318–36325 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

