The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway
- PMID: 20110357
- PMCID: PMC2843190
- DOI: 10.1074/jbc.M109.069377
The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway
Abstract
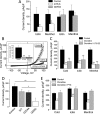
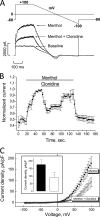
The transient receptor potential channel melastatin member 8 (TRPM8) is expressed in sensory neurons, where it constitutes the main receptor of environmental innocuous cold (10-25 degrees C). Among several types of G protein-coupled receptors expressed in sensory neurons, G(i)-coupled alpha 2A-adrenoreceptor (alpha 2A-AR), is known to be involved in thermoregulation; however, the underlying molecular mechanisms remain poorly understood. Here we demonstrated that stimulation of alpha 2A-AR inhibited TRPM8 in sensory neurons from rat dorsal root ganglia (DRG). In addition, using specific pharmacological and molecular tools combined with patch-clamp current recordings, we found that in heterologously expressed HEK-293 (human embryonic kidney) cells, TRPM8 channel is inhibited by the G(i) protein/adenylate cyclase (AC)/cAMP/protein kinase A (PKA) signaling cascade. We further identified the TRPM8 S9 and T17 as two key PKA phosphorylation sites regulating TRPM8 channel activity. We therefore propose that inhibition of TRPM8 through the alpha 2A-AR signaling cascade could constitute a new mechanism of modulation of thermosensation in both physiological and pathological conditions.
Figures


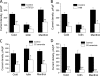


Similar articles
-
Chronic morphine regulates TRPM8 channels via MOR-PKCβ signaling.Mol Brain. 2020 Apr 14;13(1):61. doi: 10.1186/s13041-020-00599-0. Mol Brain. 2020. PMID: 32290846 Free PMC article.
-
Testosterone-androgen receptor: The steroid link inhibiting TRPM8-mediated cold sensitivity.FASEB J. 2020 Jun;34(6):7483-7499. doi: 10.1096/fj.201902270R. Epub 2020 Apr 11. FASEB J. 2020. PMID: 32277850
-
Gαq Sensitizes TRPM8 to Inhibition by PI(4,5)P2 Depletion upon Receptor Activation.J Neurosci. 2019 Jul 31;39(31):6067-6080. doi: 10.1523/JNEUROSCI.2304-18.2019. Epub 2019 May 24. J Neurosci. 2019. PMID: 31127000 Free PMC article.
-
The cool things to know about TRPM8!Channels (Austin). 2020 Dec;14(1):413-420. doi: 10.1080/19336950.2020.1841419. Channels (Austin). 2020. PMID: 33147416 Free PMC article. Review.
-
The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface.Curr Pharm Biotechnol. 2011 Jan 1;12(1):54-67. doi: 10.2174/138920111793937916. Curr Pharm Biotechnol. 2011. PMID: 20932258 Review.
Cited by
-
Trafficking of ThermoTRP Channels.Membranes (Basel). 2014 Aug 19;4(3):525-64. doi: 10.3390/membranes4030525. Membranes (Basel). 2014. PMID: 25257900 Free PMC article. Review.
-
Bidirectional modulation of thermal and chemical sensitivity of TRPM8 channels by the initial region of the N-terminal domain.J Biol Chem. 2014 Aug 8;289(32):21828-43. doi: 10.1074/jbc.M114.565994. Epub 2014 Jun 10. J Biol Chem. 2014. PMID: 24917670 Free PMC article.
-
TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1.J Cell Biol. 2017 Jul 3;216(7):2107-2130. doi: 10.1083/jcb.201506024. Epub 2017 May 26. J Cell Biol. 2017. PMID: 28550110 Free PMC article.
-
Bryostatins 1 and 3 inhibit TRPM8 and modify TRPM8- and TRPV1-mediated lung epithelial cell responses to a proinflammatory stimulus via protein kinase C.Mol Pharmacol. 2025 Jun;107(6):100042. doi: 10.1016/j.molpha.2025.100042. Epub 2025 Apr 22. Mol Pharmacol. 2025. PMID: 40378651 Free PMC article.
-
Dexmedetomidine Inhibits ASIC Activity via Activation of α2A Adrenergic Receptors in Rat Dorsal Root Ganglion Neurons.Front Pharmacol. 2021 May 24;12:685460. doi: 10.3389/fphar.2021.685460. eCollection 2021. Front Pharmacol. 2021. PMID: 34108881 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

