MEK modulates force-fluctuation-induced relengthening of canine tracheal smooth muscle
- PMID: 20110395
- PMCID: PMC3918254
- DOI: 10.1183/09031936.00160209
MEK modulates force-fluctuation-induced relengthening of canine tracheal smooth muscle
Abstract
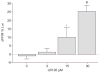
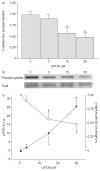
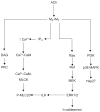
Tidal breathing, and especially deep breathing, is known to antagonise bronchoconstriction caused by airway smooth muscle (ASM) contraction; however, this bronchoprotective effect of breathing is impaired in asthma. Force fluctuations applied to contracted ASM in vitro cause it to relengthen, force-fluctuation-induced relengthening (FFIR). Given that breathing generates similar force fluctuations in ASM, FFIR represents a likely mechanism by which breathing antagonises bronchoconstriction. Thus it is of considerable interest to understand what modulates FFIR, and how ASM might be manipulated to exploit this phenomenon. It was demonstrated previously that p38 mitogen-activated protein kinase (MAPK) signalling regulates FFIR in ASM strips. Here, it was hypothesised that the MAPK kinase (MEK) signalling pathway also modulates FFIR. In order to test this hypothesis, changes in FFIR were measured in ASM treated with the MEK inhibitor, U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene). Increasing concentrations of U0126 caused greater FFIR. U0126 reduced extracellular signal-regulated kinase 1/2 phosphorylation without affecting isotonic shortening or 20-kDa myosin light chain and p38 MAPK phosphorylation. However, increasing concentrations of U0126 progressively blunted phosphorylation of high-molecular-weight caldesmon (h-caldesmon), a downstream target of MEK. Thus changes in FFIR exhibited significant negative correlation with h-caldesmon phosphorylation. The present data demonstrate that FFIR is regulated through MEK signalling, and suggest that the role of MEK is mediated, in part, through caldesmon.
Conflict of interest statement
A statement of interest for J. Solway can be found at
Figures


Similar articles
-
Steroids augment relengthening of contracted airway smooth muscle: potential additional mechanism of benefit in asthma.Eur Respir J. 2008 Nov;32(5):1224-30. doi: 10.1183/09031936.00092908. Epub 2008 Sep 3. Eur Respir J. 2008. PMID: 18768574 Free PMC article.
-
Force fluctuation-induced relengthening of acetylcholine-contracted airway smooth muscle.Proc Am Thorac Soc. 2008 Jan 1;5(1):68-72. doi: 10.1513/pats.200705-058VS. Proc Am Thorac Soc. 2008. PMID: 18094087 Free PMC article. Review.
-
Latrunculin B increases force fluctuation-induced relengthening of ACh-contracted, isotonically shortened canine tracheal smooth muscle.J Appl Physiol (1985). 2005 Feb;98(2):489-97. doi: 10.1152/japplphysiol.01378.2003. Epub 2004 Oct 1. J Appl Physiol (1985). 2005. PMID: 15465883
-
Disrupting actin-myosin-actin connectivity in airway smooth muscle as a treatment for asthma?Proc Am Thorac Soc. 2009 May 1;6(3):295-300. doi: 10.1513/pats.200808-078RM. Proc Am Thorac Soc. 2009. PMID: 19387033 Free PMC article. Review.
-
Identification of a novel inhibitor of mitogen-activated protein kinase kinase.J Biol Chem. 1998 Jul 17;273(29):18623-32. doi: 10.1074/jbc.273.29.18623. J Biol Chem. 1998. PMID: 9660836
Cited by
-
Dilatation of the constricted human airway by tidal expansion of lung parenchyma.Am J Respir Crit Care Med. 2012 Aug 1;186(3):225-32. doi: 10.1164/rccm.201202-0368OC. Epub 2012 Jun 7. Am J Respir Crit Care Med. 2012. PMID: 22679010 Free PMC article.
-
Airway smooth muscle in the pathophysiology and treatment of asthma.J Appl Physiol (1985). 2013 Apr;114(7):834-43. doi: 10.1152/japplphysiol.00950.2012. Epub 2013 Jan 10. J Appl Physiol (1985). 2013. PMID: 23305987 Free PMC article. Review.
-
A life off the beaten track in biomechanics: Imperfect elasticity, cytoskeletal glassiness, and epithelial unjamming.Biophys Rev (Melville). 2023 Dec;4(4):041304. doi: 10.1063/5.0179719. Epub 2023 Dec 21. Biophys Rev (Melville). 2023. PMID: 38156333 Free PMC article. Review.
-
Emergence of airway smooth muscle functions related to structural malleability.J Appl Physiol (1985). 2011 Apr;110(4):1130-5. doi: 10.1152/japplphysiol.01192.2010. Epub 2010 Dec 2. J Appl Physiol (1985). 2011. PMID: 21127211 Free PMC article. Review.
-
The Strain on Airway Smooth Muscle During a Deep Inspiration to Total Lung Capacity.J Eng Sci Med Diagn Ther. 2019 Feb;2(1):0108021-1080221. doi: 10.1115/1.4042309. Epub 2019 Jan 18. J Eng Sci Med Diagn Ther. 2019. PMID: 32328568 Free PMC article.
References
-
- Fredberg JJ, Inouye D, Miller B, et al. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med. 1997;156:1752–1759. - PubMed
-
- Fredberg JJ, Inouye DS, Mijailovich SM, et al. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med. 1999;159:959–967. - PubMed
-
- Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
