Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy
- PMID: 20110406
- PMCID: PMC2832170
- DOI: 10.2353/ajpath.2010.090398
Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy
Abstract
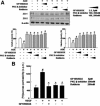
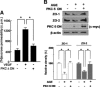
Vision loss in diabetic retinopathy is due to macular edema characterized by increased vascular permeability, which involves phosphorylation associated with activation of protein kinase C (PKC) isoforms. Herein, we demonstrated PKC delta inhibition could prevent blood-retinal barrier breakdown in diabetic retinopathy. Increased vascular permeability of diabetic retina was accompanied by a decrease of zonula occludens (ZO)-1 and ZO-2 expression. In diabetic retina and advanced glycation end product-treated human retinal microvascular endothelial cells, vascular leakage and loss of ZO-1 and ZO-2 on retinal vessels were effectively restored or prevented with treatment of rottlerin, transfection of PKC-delta-DN, or siRNA for PKC delta. Interestingly, PKC delta translocated from cytosol to membrane in advanced glycation end product-treated human retinal microvascular endothelial cells, which was blocked by PKC delta inhibition. Taken together, PKC delta activation, related to its subcellular translocation, is involved in vascular permeability in response to diabetes, and inhibition of PKC delta effectively restores loss of tight junction proteins in retinal vessels. Therefore, we suggest that inhibition of PKC delta could be an alternative treatment to blood-retinal barrier breakdown in diabetic retinopathy.
Figures




References
-
- Porta M, Bandello F. Diabetic retinopathy: an update. Diabetologia. 2002;45:1617–1634. - PubMed
-
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. - PubMed
-
- Davidson JA, Ciulla TA, McGill JB, Kles KA, Anderson PW. How the diabetic eye loses vision. Endocrine. 2007;32:107–116. - PubMed
-
- Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. - PubMed
-
- Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia. 2001;44:791–804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

