Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers
- PMID: 20110408
- PMCID: PMC2832148
- DOI: 10.2353/ajpath.2010.090527
Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers
Abstract
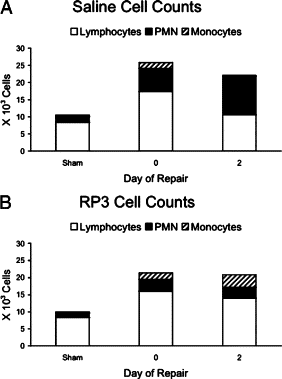
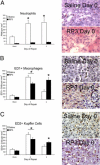
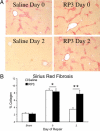
Biliary obstruction results in a well-characterized cholestatic inflammatory and fibrogenic process; however, the mechanisms and potential for liver repair remain unclear. We previously demonstrated that Kupffer cell depletion reduces polymorphonuclear cell (neutrophil) (PMN) and matrix metalloproteinase (MMP)8 levels in repairing liver. We therefore hypothesized that PMN-dependent MMP activity is essential for successful repair. Male Sprague-Dawley rats received reversible biliary obstruction for 7 days, and the rat PMN-specific antibody RP3 was administered 2 days before biliary decompression (repair) and continued daily until necropsy, when liver underwent morphometric analysis, immunohistochemistry, quantitative RT-PCR, and in situ zymography. We found that RP3 treatment did not reduce Kupffer cell or monocyte number but significantly reduced PMN number at the time of decompression and 2 days after repair. RP3 treatment also blocked resorption of type I collagen. In addition, biliary obstruction resulted in increased expression of MMP3, MMP8, and tissue inhibitor of metalloproteinase 1. Two days after biliary decompression, both MMP3 and tissue inhibitor of metalloproteinase 1 expression declined toward sham levels, whereas MMP8 expression remained elevated and was identified in bile duct epithelial cells by immunohistochemistry. PMN depletion did not alter the hepatic expression of these genes. Conversely, collagen-based in situ zymography demonstrated markedly diminished collagenase activity following PMN depletion. We conclude that PMNs are essential for collagenase activity and collagen resorption during liver repair, and speculate that PMN-derived MMP8 or PMN-mediated activation of intrinsic hepatic MMPs are responsible for successful liver repair.
Figures



Similar articles
-
Repair after cholestatic liver injury correlates with neutrophil infiltration and matrix metalloproteinase 8 activity.Surgery. 2005 Aug;138(2):313-20. doi: 10.1016/j.surg.2005.04.009. Surgery. 2005. PMID: 16153442
-
Dexamethasone alters the hepatic inflammatory cellular profile without changes in matrix degradation during liver repair following biliary decompression.J Surg Res. 2009 Oct;156(2):231-9. doi: 10.1016/j.jss.2009.04.016. Epub 2009 May 14. J Surg Res. 2009. PMID: 19592011 Free PMC article.
-
Hepatic macrophages promote the neutrophil-dependent resolution of fibrosis in repairing cholestatic rat livers.Surgery. 2008 May;143(5):667-78. doi: 10.1016/j.surg.2008.01.008. Surgery. 2008. PMID: 18436015
-
Bone marrow mononuclear cell transplantation increases metalloproteinase-9 and 13 and decreases tissue inhibitors of metalloproteinase-1 and 2 expression in the liver of cholestatic rats.Cells Tissues Organs. 2013;198(2):139-48. doi: 10.1159/000353215. Epub 2013 Jul 24. Cells Tissues Organs. 2013. PMID: 23886643
-
Regulation of collagen degradation in the rat myocardium after infarction.J Mol Cell Cardiol. 1995 Jun;27(6):1281-92. doi: 10.1016/s0022-2828(05)82390-9. J Mol Cell Cardiol. 1995. PMID: 8531210
Cited by
-
Neutrophil roles in left ventricular remodeling following myocardial infarction.Fibrogenesis Tissue Repair. 2013 Jun 3;6(1):11. doi: 10.1186/1755-1536-6-11. Fibrogenesis Tissue Repair. 2013. PMID: 23731794 Free PMC article.
-
Repair after acute lung injury: molecular mechanisms and therapeutic opportunities.Crit Care. 2012 Dec 12;16(2):209. doi: 10.1186/cc11224. Crit Care. 2012. PMID: 22429641 Free PMC article. Review. No abstract available.
-
Matrix metalloproteinase-9, -10, and -12, MDM2 and p53 expression in mouse liver during dimethylnitrosamine-induced oxidative stress and genomic injury.Mol Cell Biochem. 2012 Jun;365(1-2):351-61. doi: 10.1007/s11010-012-1277-z. Epub 2012 Mar 23. Mol Cell Biochem. 2012. PMID: 22441882
-
Selective inhibitor of Wnt/β-catenin/CBP signaling ameliorates hepatitis C virus-induced liver fibrosis in mouse model.Sci Rep. 2017 Mar 23;7(1):325. doi: 10.1038/s41598-017-00282-w. Sci Rep. 2017. PMID: 28336942 Free PMC article.
-
Human Neutrophil α-Defensins 1-3 Are Upregulated in the Microenvironment of Fibrotic Liver.Medicina (Kaunas). 2023 Mar 2;59(3):496. doi: 10.3390/medicina59030496. Medicina (Kaunas). 2023. PMID: 36984497 Free PMC article.
References
-
- Tracy TF, Jr, Goerke ME, Bailey PV, Sotelo-Avila C, Weber TR. Growth-related gene expression in early cholestatic liver injury. Surgery. 1993;114:532–537. - PubMed
-
- Slott PA, Liu MH, Tavoloni N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology. 1990;99:466–477. - PubMed
-
- Roggin KK, Kim JC, Kurkchubasche AG, Papa EF, Vezeridis AM, Tracy TF. Macrophage phenotype during cholestatic injury and repair: the persistent inflammatory response. J Pediatr Surg. 2001;36:220–228. - PubMed
-
- Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. - PubMed
-
- Saito JM, Bostick MK, Campe CB, Xu J, Maher JJ. Infiltrating neutrophils in bile duct-ligated livers do not promote hepatic fibrosis. Hepatol Res. 2003;25:180–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

