COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems
- PMID: 20110411
- PMCID: PMC2832166
- DOI: 10.2353/ajpath.2010.090607
COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems
Abstract
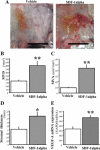
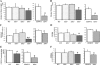
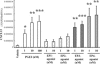
Bone marrow (BM)-derived hematopoietic cells, which are major components of tumor stroma, determine the tumor microenvironment and regulate tumor phenotypes. Cyclooxygenase (COX)-2 and endogenous prostaglandins are important determinants for tumor growth and tumor-associated angiogenesis; however, their contributions to stromal formation and angiogenesis remain unclear. In this study, we observed that Lewis lung carcinoma cells implanted in wild-type mice formed a tumor mass with extensive stromal formation that was markedly suppressed by COX-2 inhibition, which reduced the recruitment of BM cells. Notably, COX-2 inhibition attenuated CXCL12/CXCR4 expression as well as expression of several other chemokines. Indeed, in a Matrigel model, prostaglandin (PG) E2 enhanced stromal formation and CXCL12/CXCR4 expression. In addition, a COX-2 inhibitor suppressed stromal formation and reduced expression of CXCL12/CXCR4 and a fibroblast marker (S100A4) in a micropore chamber model. Moreover, stromal formation after tumor implantation was suppressed in EP3-/- mice and EP4-/- mice, in which stromal expression of CXCL12/CXCR4 and S100A4 was reduced. The EP3 or EP4 knockout suppressed S100A4+ fibroblasts, CXCL12+, and/or CXCR4+ stromal cells as well. Immunofluorescent analyses revealed that CXCL12+CXCR4+S100A4+ fibroblasts mainly comprised stromal cells and most of these were recruited from the BM. Additionally, either EP3- or EP4-specific agonists stimulated CXCL12 expression by fibroblasts in vitro. The present results address the novel activities of COX-2/PGE2-EP3/EP4 signaling that modulate tumor biology and show that CXCL12/CXCR4 axis may play a crucial role in tumor stromal formation and angiogenesis under the control of prostaglandins.
Figures




Similar articles
-
Roles of prostaglandin E2-EP3/EP4 receptor signaling in the enhancement of lymphangiogenesis during fibroblast growth factor-2-induced granulation formation.Arterioscler Thromb Vasc Biol. 2011 May;31(5):1049-58. doi: 10.1161/ATVBAHA.110.222356. Epub 2011 Feb 10. Arterioscler Thromb Vasc Biol. 2011. PMID: 21311040
-
Recruited bone marrow cells expressing the EP3 prostaglandin E receptor subtype enhance angiogenesis during chronic inflammation.Biomed Pharmacother. 2010 Feb;64(2):93-100. doi: 10.1016/j.biopha.2009.04.034. Epub 2009 Oct 17. Biomed Pharmacother. 2010. PMID: 20015609
-
Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth.J Exp Med. 2003 Jan 20;197(2):221-32. doi: 10.1084/jem.20021408. J Exp Med. 2003. PMID: 12538661 Free PMC article.
-
Targeting SDF-1 in multiple myeloma tumor microenvironment.Cancer Lett. 2016 Sep 28;380(1):315-8. doi: 10.1016/j.canlet.2015.11.028. Epub 2015 Nov 30. Cancer Lett. 2016. PMID: 26655999 Review.
-
CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment.Blood. 2006 Mar 1;107(5):1761-7. doi: 10.1182/blood-2005-08-3182. Epub 2005 Nov 3. Blood. 2006. PMID: 16269611 Review.
Cited by
-
Prostaglandin E2 regulates pancreatic stellate cell activity via the EP4 receptor.Pancreas. 2013 Apr;42(3):467-74. doi: 10.1097/MPA.0b013e318264d0f8. Pancreas. 2013. PMID: 23090667 Free PMC article.
-
Prostaglandins in cancer cell adhesion, migration, and invasion.Int J Cell Biol. 2012;2012:723419. doi: 10.1155/2012/723419. Epub 2012 Feb 29. Int J Cell Biol. 2012. PMID: 22505934 Free PMC article.
-
Functional heterogeneity of breast fibroblasts is defined by a prostaglandin secretory phenotype that promotes expansion of cancer-stem like cells.PLoS One. 2011;6(9):e24605. doi: 10.1371/journal.pone.0024605. Epub 2011 Sep 21. PLoS One. 2011. PMID: 21957456 Free PMC article.
-
Apricoxib, a novel inhibitor of COX-2, markedly improves standard therapy response in molecularly defined models of pancreatic cancer.Clin Cancer Res. 2012 Sep 15;18(18):5031-42. doi: 10.1158/1078-0432.CCR-12-0453. Epub 2012 Jul 24. Clin Cancer Res. 2012. PMID: 22829202 Free PMC article.
-
Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation.Reprod Biol Endocrinol. 2018 May 1;16(1):43. doi: 10.1186/s12958-018-0359-5. Reprod Biol Endocrinol. 2018. PMID: 29716588 Free PMC article. Review.
References
-
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

