Pathology of gastrointestinal organs in a porcine model of cystic fibrosis
- PMID: 20110417
- PMCID: PMC2832157
- DOI: 10.2353/ajpath.2010.090849
Pathology of gastrointestinal organs in a porcine model of cystic fibrosis
Abstract
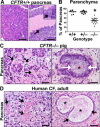
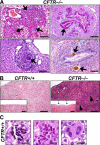
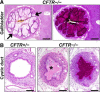
Cystic fibrosis (CF), which is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), is characterized by multiorgan pathology that begins early in life. To better understand the initial stages of disease, we studied the gastrointestinal pathology of CFTR-/- pigs. By studying newborns, we avoided secondary changes attributable to environmental interactions, infection, or disease progression. Lesions resembling those in humans with CF were detected in intestine, pancreas, liver, gallbladder, and cystic duct. These organs had four common features. First, disease was accelerated compared with that in humans, which could provide a strategy to discover modifying factors. Second, affected organs showed variable hyperplastic, metaplastic, and connective tissue changes, indicating that remodeling was a dynamic component of fetal life. Third, cellular inflammation was often mild to moderate and not always present, which raises new questions as to the role of cellular inflammation in early disease pathogenesis. Fourth, epithelial mucus-producing cells were often increased, producing a striking accumulation of mucus with a layered appearance and resilient structure. Thus, mucus cell hyperplasia and mucus accumulation play prominent roles in early disease. Our findings also have implications for CF lung disease, and they lay the foundation for a better understanding of CF pathogenesis.
Figures






Similar articles
-
Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets.Am J Pathol. 2014 May;184(5):1309-22. doi: 10.1016/j.ajpath.2014.01.035. Epub 2014 Mar 15. Am J Pathol. 2014. PMID: 24637292 Free PMC article.
-
Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model.Am J Pathol. 2004 Apr;164(4):1481-93. doi: 10.1016/S0002-9440(10)63234-8. Am J Pathol. 2004. PMID: 15039235 Free PMC article.
-
Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis.J Mol Med (Berl). 2012 May;90(5):597-608. doi: 10.1007/s00109-011-0839-y. Epub 2011 Dec 15. J Mol Med (Berl). 2012. PMID: 22170306
-
Bicarbonate in cystic fibrosis.J Cyst Fibros. 2017 Nov;16(6):653-662. doi: 10.1016/j.jcf.2017.06.005. Epub 2017 Jul 18. J Cyst Fibros. 2017. PMID: 28732801 Review.
-
Cystic fibrosis: an inherited disease affecting mucin-producing organs.Int J Biochem Cell Biol. 2014 Jul;52:136-45. doi: 10.1016/j.biocel.2014.03.011. Epub 2014 Mar 28. Int J Biochem Cell Biol. 2014. PMID: 24685676 Free PMC article. Review.
Cited by
-
Influences of exocrine pancreatic insufficiency on nutrient digestibility, growth parameters as well as anatomical and histological morphology of the intestine in a juvenile pig model.Front Med (Lausanne). 2022 Sep 9;9:973589. doi: 10.3389/fmed.2022.973589. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36160141 Free PMC article.
-
A Developmental Role of the Cystic Fibrosis Transmembrane Conductance Regulator in Cystic Fibrosis Lung Disease Pathogenesis.Front Cell Dev Biol. 2021 Oct 11;9:742891. doi: 10.3389/fcell.2021.742891. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34708042 Free PMC article. Review.
-
A porcine model of neurofibromatosis type 1 that mimics the human disease.JCI Insight. 2018 Jun 21;3(12):e120402. doi: 10.1172/jci.insight.120402. eCollection 2018 Jun 21. JCI Insight. 2018. PMID: 29925695 Free PMC article.
-
New animal models of cystic fibrosis: what are they teaching us?Curr Opin Pulm Med. 2011 Nov;17(6):478-83. doi: 10.1097/MCP.0b013e32834b14c9. Curr Opin Pulm Med. 2011. PMID: 21857224 Free PMC article. Review.
-
Lessons learned from the cystic fibrosis pig.Theriogenology. 2016 Jul 1;86(1):427-32. doi: 10.1016/j.theriogenology.2016.04.057. Epub 2016 Apr 21. Theriogenology. 2016. PMID: 27142487 Free PMC article. Review.
References
-
- Landsteiner K. Darmverschluss durch eingedictes Meconium Pankreatitis. Zentralbl Allg Pathol. 1905;16:903–907.
-
- Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344–399.
-
- Farber S. Pancreatic function and disease early in life V. Pathologic changes associated with pancreatic insufficiency in early life. Arch Path. 1944;37:238–250.
-
- Farber S. Some organic digestive disturbances early in life. J Mich Med Soc. 1946;44:587–594.
-
- Bodian M. Fibrocystic Disease of the Pancreas: a Congenital Disorder of Mucus Production-Mucosis. Grune & Stratton; New York, NY: 1953. pp. 67–146.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

