An in vivo model to study and manipulate the hematopoietic stem cell niche
- PMID: 20110425
- PMCID: PMC2852363
- DOI: 10.1182/blood-2009-01-200071
An in vivo model to study and manipulate the hematopoietic stem cell niche
Abstract
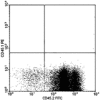
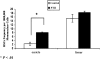
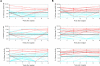
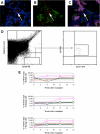
Because the microenvironment that supports hematopoietic stem cell (HSC) proliferation and differentiation is not fully understood, we adapted a heterotopic bone formation model as a new approach for studying the HSC microenvironment in vivo. Endogenous HSCs homed to tissue-engineered ossicles and individually sorted HSCs from ossicles were able to reconstitute lethally irradiated mice. To further explore this model as a system to study the stem cell niche, ossicles were established with or without anabolic parathyroid hormone (PTH) treatment during the 4-week course of bone development. Histology and micro-computed tomography showed higher bone area-to-total area ratios, thicker cortical bone and trabecular bone, significantly higher bone mineral density and bone volume fraction in PTH-treated groups than in controls. By an in vivo competitive long-term reconstitution assay, HSC frequency in the ossicle marrow was 3 times greater in PTH groups than in controls. When whole bone marrow cells were directly injected into the ossicles after lethal irradiation, the PTH-treated groups showed an enhanced reconstitution rate compared with controls. These findings suggest the residence of HSCs in heterotopic bone marrow and support the future use of this ossicle model in elucidating the composition and regulation of the HSC niche.
Figures

Similar articles
-
Intramarrow injection of beta-catenin-activated, but not naive mesenchymal stromal cells stimulates self-renewal of hematopoietic stem cells in bone marrow.Exp Mol Med. 2010 Feb 28;42(2):122-31. doi: 10.3858/emm.2010.42.2.014. Exp Mol Med. 2010. PMID: 20054234 Free PMC article.
-
Human umbilical cord blood-borne fibroblasts contain marrow niche precursors that form a bone/marrow organoid in vivo.Development. 2017 Mar 15;144(6):1035-1044. doi: 10.1242/dev.142836. Development. 2017. PMID: 28292847 Free PMC article.
-
An ectopic stromal implant model for hematopoietic reconstitution and in vivo evaluation of bone marrow niches.Cell Transplant. 2012;21(12):2677-88. doi: 10.3727/096368912X636993. Epub 2012 Apr 2. Cell Transplant. 2012. PMID: 22472430
-
Role of the microenvironment of the embryonic aorta-gonad-mesonephros region in hematopoiesis.Ann N Y Acad Sci. 2001 Jun;938:109-16. doi: 10.1111/j.1749-6632.2001.tb03579.x. Ann N Y Acad Sci. 2001. PMID: 11458497 Review.
-
Parathyroid hormone: a novel tool for treating bone marrow depletion in cancer patients caused by chemotherapeutic drugs and ionizing radiation.Cancer Lett. 2006 Nov 28;244(1):8-15. doi: 10.1016/j.canlet.2006.02.006. Epub 2006 Mar 15. Cancer Lett. 2006. PMID: 16540235 Review.
Cited by
-
Ex Vivo Expansion of Hematopoietic Stem Cells for Therapeutic Purposes: Lessons from Development and the Niche.Cells. 2019 Feb 18;8(2):169. doi: 10.3390/cells8020169. Cells. 2019. PMID: 30781676 Free PMC article. Review.
-
Dynamic niches in the origination and differentiation of haematopoietic stem cells.Nat Rev Mol Cell Biol. 2011 Sep 2;12(10):643-55. doi: 10.1038/nrm3184. Nat Rev Mol Cell Biol. 2011. PMID: 21886187 Free PMC article. Review.
-
A T Cell View of the Bone Marrow.Front Immunol. 2016 May 17;7:184. doi: 10.3389/fimmu.2016.00184. eCollection 2016. Front Immunol. 2016. PMID: 27242791 Free PMC article.
-
Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment.Neoplasia. 2012 May;14(5):429-39. doi: 10.1596/neo.111740. Neoplasia. 2012. PMID: 22745589 Free PMC article.
-
Human early fracture hematoma is characterized by inflammation and hypoxia.Clin Orthop Relat Res. 2011 Nov;469(11):3118-26. doi: 10.1007/s11999-011-1865-3. Clin Orthop Relat Res. 2011. PMID: 21409457 Free PMC article.
References
-
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. - PubMed
-
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–1074. - PubMed
-
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. - PubMed
-
- Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

