AE2 Cl-/HCO3- exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon
- PMID: 20110461
- PMCID: PMC2853300
- DOI: 10.1152/ajpgi.00178.2009
AE2 Cl-/HCO3- exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon
Abstract
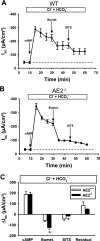
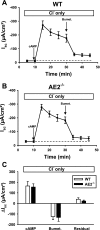
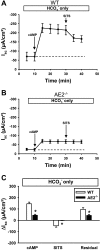
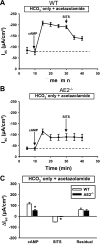
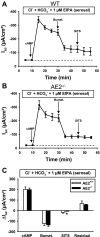
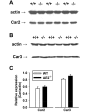
Anion secretion by colonic epithelium is dependent on apical CFTR-mediated anion conductance and basolateral ion transport. In many tissues, the NKCC1 Na(+)-K(+)-2Cl(-) cotransporter mediates basolateral Cl(-) uptake. However, additional evidence suggests that the AE2 Cl(-)/HCO(3)(-) exchanger, when coupled with the NHE1 Na(+)/H(+) exchanger or a Na(+)-HCO(3)(-) cotransporter (NBC), contributes to HCO(3)(-) and/or Cl(-) uptake. To analyze the secretory functions of AE2 in proximal colon, short-circuit current (I(sc)) responses to cAMP and inhibitors of basolateral anion transporters were measured in muscle-stripped wild-type (WT) and AE2-null (AE2(-/-)) proximal colon. In physiological Ringer, the magnitude of cAMP-stimulated I(sc) was the same in WT and AE2(-/-) colon. However, the I(sc) response in AE2(-/-) colon exhibited increased sensitivity to the NKCC1 inhibitor bumetanide and decreased sensitivity to the distilbene derivative SITS (which inhibits AE2 and some NBCs), indicating that loss of AE2 results in a switch to increased NKCC1-supported anion secretion. Removal of HCO(3)(-) resulted in robust cAMP-stimulated I(sc) in both AE2(-/-) and WT colon that was largely mediated by NKCC1, whereas removal of Cl(-) resulted in sharply decreased cAMP-stimulated I(sc) in AE2(-/-) colon relative to WT controls. Inhibition of NHE1 had no effect on cAMP-stimulated I(sc) in AE2(-/-) colon but caused a switch to NKCC1-supported secretion in WT colon. Thus, in AE2(-/-) colon, Cl(-) secretion supported by basolateral NKCC1 is enhanced, whereas HCO(3)(-) secretion is diminished. These results show that AE2 is a component of the basolateral ion transport mechanisms that support anion secretion in the proximal colon.
Figures


Similar articles
-
Basolateral chloride loading by the anion exchanger type 2: role in fluid secretion by the human airway epithelial cell line Calu-3.J Physiol. 2012 Nov 1;590(21):5299-316. doi: 10.1113/jphysiol.2012.236919. Epub 2012 Jul 16. J Physiol. 2012. PMID: 22802585 Free PMC article.
-
Bicarbonate secretion by rat bile duct brush cells indicated by immunohistochemical localization of CFTR, anion exchanger AE2, Na+/HCO3 -cotransporter, carbonic anhydrase II, Na+/H+ exchangers NHE1 and NHE3, H+/K+-ATPase, and Na+/K+-ATPase.Med Mol Morphol. 2006 Mar;39(1):44-8. doi: 10.1007/s00795-006-0312-0. Med Mol Morphol. 2006. PMID: 16575514
-
An alternate pathway of cAMP-stimulated Cl secretion across the NKCC1-null murine duodenum.Gastroenterology. 2002 Aug;123(2):531-41. doi: 10.1053/gast.2002.34757. Gastroenterology. 2002. PMID: 12145806
-
Molecular physiology of SLC4 anion exchangers.Exp Physiol. 2006 Jan;91(1):153-61. doi: 10.1113/expphysiol.2005.031765. Epub 2005 Oct 20. Exp Physiol. 2006. PMID: 16239253 Review.
-
Mechanisms of bicarbonate secretion: lessons from the airways.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a015016. doi: 10.1101/cshperspect.a015016. Cold Spring Harb Perspect Med. 2012. PMID: 22908201 Free PMC article. Review.
Cited by
-
Critical role of bicarbonate and bicarbonate transporters in cardiac function.World J Biol Chem. 2014 Aug 26;5(3):334-45. doi: 10.4331/wjbc.v5.i3.334. World J Biol Chem. 2014. PMID: 25225601 Free PMC article. Review.
-
Membrane Transport Proteins in Osteoclasts: The Ins and Outs.Front Cell Dev Biol. 2021 Feb 26;9:644986. doi: 10.3389/fcell.2021.644986. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718388 Free PMC article. Review.
-
Gastrin inhibits a novel, pathological colon cancer signaling pathway involving EGR1, AE2, and P-ERK.J Mol Med (Berl). 2012 Jun;90(6):707-18. doi: 10.1007/s00109-011-0851-2. Epub 2012 Jan 7. J Mol Med (Berl). 2012. PMID: 22228178 Free PMC article.
-
The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters.Physiol Rev. 2013 Apr;93(2):803-959. doi: 10.1152/physrev.00023.2012. Physiol Rev. 2013. PMID: 23589833 Free PMC article. Review.
-
Ae4 (Slc4a9) Anion Exchanger Drives Cl- Uptake-dependent Fluid Secretion by Mouse Submandibular Gland Acinar Cells.J Biol Chem. 2015 Apr 24;290(17):10677-88. doi: 10.1074/jbc.M114.612895. Epub 2015 Mar 5. J Biol Chem. 2015. PMID: 25745107 Free PMC article.
References
-
- Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 187: 159–167, 2006 - PubMed
-
- Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G332, 1999 - PubMed
-
- Alrefai WA, Tyagi S, Nazir TM, Barakat J, Anwar SS, Hadjiagapiou C, Bavishi D, Sahi J, Malik P, Goldstein J, Layden TJ, Ramaswamy K, Dudeja PK. Human intestinal anion exchanger isoforms: expression, distribution, and membrane localization. Biochim Biophys Acta 1511: 17–27, 2001 - PubMed
-
- Alvarez BV, Kieller DM, Quon AL, Robertson M, Casey JR. Cardiac hypertrophy in anion exchanger 1-null mutant mice with severe hemolytic anemia. Am J Physiol Heart Circ Physiol 292: H1301–H1312, 2007 - PubMed
-
- Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+-HCO3− cotransport activity in murine colonic crypts in a M3-, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol 291: G650–G657, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

