miR-221 suppresses ICAM-1 translation and regulates interferon-gamma-induced ICAM-1 expression in human cholangiocytes
- PMID: 20110463
- PMCID: PMC2853302
- DOI: 10.1152/ajpgi.00490.2009
miR-221 suppresses ICAM-1 translation and regulates interferon-gamma-induced ICAM-1 expression in human cholangiocytes
Abstract
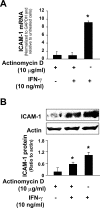
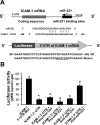
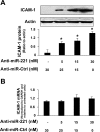
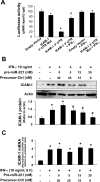
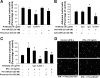
Aberrant cholangiocyte reactions in response to inflammatory stimuli are important pathogenic factors for the persistent biliary inflammation in patients with cholangiopathies. Overexpression of intercellular cell adhesion molecule-1 (ICAM-1) in cholangiocytes is a common pathological feature in inflammatory cholangiopathies and can promote cholangiocyte interactions with effector lymphocytes in the portal region. In this study, we tested the involvement of miRNA-mediated posttranscriptional regulation in IFN-gamma-induced ICAM-1 expression in cholangiocytes. Using both immortalized and nonimmortalized human cholangiocyte cell lines, we found that IFN-gamma activated ICAM-1 transcription and increased ICAM-1 protein expression. Inhibition of ICAM-1 transcription could only partially block IFN-gamma-induced ICAM-1 expression at the protein level. In silico target prediction analysis revealed complementarity of miR-221 to the 3'-untranslated region of ICAM-1 mRNA. Targeting of ICAM-1 3'-untranslated region by miR-221 resulted in translational repression in cholangiocytes but not ICAM-1 mRNA degradation. Functional inhibition of miR-221 with anti-miR-221 induced ICAM-1 protein expression. Moreover, IFN-gamma stimulation decreased miR-221 expression in cholangiocytes in a signal transducer and activator of transcription 1-dependent manner. Transfection of miR-221 precursor abolished IFN-gamma-stimulated ICAM-1 protein expression. In addition, miR-221-mediated expression of ICAM-1 on cholangiocytes showed a significant influence on the adherence of cocultured T cells. These findings indicate that both transcriptional and miRNA-mediated posttranscriptional mechanisms are involved in IFN-gamma-induced ICAM-1 expression in human cholangiocytes, suggesting an important role for miRNAs in the regulation of cholangiocyte inflammatory responses.
Figures

Similar articles
-
MicroRNA-221 controls expression of intercellular adhesion molecule-1 in epithelial cells in response to Cryptosporidium parvum infection.Int J Parasitol. 2011 Mar;41(3-4):397-403. doi: 10.1016/j.ijpara.2010.11.011. Epub 2011 Jan 12. Int J Parasitol. 2011. PMID: 21236259 Free PMC article.
-
MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes.J Immunol. 2009 Feb 1;182(3):1325-33. doi: 10.4049/jimmunol.182.3.1325. J Immunol. 2009. PMID: 19155478 Free PMC article.
-
Pine bark extract pycnogenol downregulates IFN-gamma-induced adhesion of T cells to human keratinocytes by inhibiting inducible ICAM-1 expression.Free Radic Biol Med. 2000 Jan 15;28(2):219-27. doi: 10.1016/s0891-5849(99)00229-4. Free Radic Biol Med. 2000. PMID: 11281289
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
The immunobiology of cholangiocytes.Immunol Cell Biol. 2008 Aug-Sep;86(6):497-505. doi: 10.1038/icb.2008.37. Epub 2008 May 27. Immunol Cell Biol. 2008. PMID: 18504452 Free PMC article. Review.
Cited by
-
Roles of intercellular cell adhesion molecule-1 (ICAM-1) in colorectal cancer: expression, functions, prognosis, tumorigenesis, polymorphisms and therapeutic implications.Front Oncol. 2022 Nov 23;12:1052672. doi: 10.3389/fonc.2022.1052672. eCollection 2022. Front Oncol. 2022. PMID: 36505809 Free PMC article. Review.
-
Human miR-221/222 in Physiological and Atherosclerotic Vascular Remodeling.Biomed Res Int. 2015;2015:354517. doi: 10.1155/2015/354517. Epub 2015 Jun 29. Biomed Res Int. 2015. PMID: 26221589 Free PMC article. Review.
-
Variable Resistance of RMS to Interferon γ Signaling.ISRN Oncol. 2012;2012:789152. doi: 10.5402/2012/789152. Epub 2012 Aug 5. ISRN Oncol. 2012. PMID: 22919516 Free PMC article.
-
A novel immune resistance mechanism of melanoma cells controlled by the ADAR1 enzyme.Oncotarget. 2015 Oct 6;6(30):28999-9015. doi: 10.18632/oncotarget.4905. Oncotarget. 2015. PMID: 26338962 Free PMC article.
-
MicroRNA-221 controls expression of intercellular adhesion molecule-1 in epithelial cells in response to Cryptosporidium parvum infection.Int J Parasitol. 2011 Mar;41(3-4):397-403. doi: 10.1016/j.ijpara.2010.11.011. Epub 2011 Jan 12. Int J Parasitol. 2011. PMID: 21236259 Free PMC article.
References
-
- Alpini G, McGill JM, LaRusso NF. The pathobiology of biliary epithelia. Hepatology 35: 1256–1268, 2002 - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004 - PubMed
-
- Aoki CA, Bowlus CL, Gershwin ME. The immunobiology of primary sclerosing cholangitis. Autoimmun Rev 4: 137–143, 2005 - PubMed
-
- Arnold R, Konig W. Respiratory syncytial virus infection of human lung endothelial cells enhances selectively intercellular adhesion molecule-1 expression. J Immunol 174: 7359–7367, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

