Generating a prion with bacterially expressed recombinant prion protein
- PMID: 20110469
- PMCID: PMC2893558
- DOI: 10.1126/science.1183748
Generating a prion with bacterially expressed recombinant prion protein
Abstract
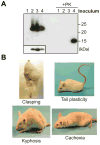
The prion hypothesis posits that a misfolded form of prion protein (PrP) is responsible for the infectivity of prion disease. Using recombinant murine PrP purified from Escherichia coli, we created a recombinant prion with the attributes of the pathogenic PrP isoform: aggregated, protease-resistant, and self-perpetuating. After intracerebral injection of the recombinant prion, wild-type mice developed neurological signs in approximately 130 days and reached the terminal stage of disease in approximately 150 days. Characterization of diseased mice revealed classic neuropathology of prion disease, the presence of protease-resistant PrP, and the capability of serially transmitting the disease; these findings confirmed that the mice succumbed to prion disease. Thus, as postulated by the prion hypothesis, the infectivity in mammalian prion disease results from an altered conformation of PrP.
Figures


Comment in
-
Biochemistry. What makes a prion infectious?Science. 2010 Feb 26;327(5969):1091-2. doi: 10.1126/science.1187790. Science. 2010. PMID: 20185716 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

