Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice
- PMID: 20110532
- PMCID: PMC4317351
- DOI: 10.1161/CIRCRESAHA.109.210682
Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice
Abstract
Rationale: Multiple biological mechanisms contribute to the efficacy of cardiac cell therapy. Most prominent among these are direct heart muscle and blood vessel regeneration from transplanted cells, as opposed to paracrine enhancement of tissue preservation and/or recruitment of endogenous repair.
Objective: Human cardiac progenitor cells, cultured as cardiospheres (CSps) or as CSp-derived cells (CDCs), have been shown to be capable of direct cardiac regeneration in vivo. Here we characterized paracrine effects in CDC transplantation and investigated their relative importance versus direct differentiation of surviving transplanted cells.
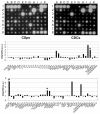
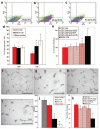
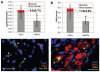
Methods and results: In vitro, many growth factors were found in media conditioned by human adult CSps and CDCs; CDC-conditioned media exerted antiapoptotic effects on neonatal rat ventricular myocytes, and proangiogenic effects on human umbilical vein endothelial cells. In vivo, human CDCs secreted vascular endothelial growth factor, hepatocyte growth factor, and insulin-like growth factor 1 when transplanted into the same SCID mouse model of acute myocardial infarction where they were previously shown to improve function and to produce tissue regeneration. Injection of CDCs in the peri-infarct zone increased the expression of Akt, decreased apoptotic rate and caspase 3 level, and increased capillary density, indicating overall higher tissue resilience. Based on the number of human-specific cells relative to overall increases in capillary density and myocardial viability, direct differentiation quantitatively accounted for 20% to 50% of the observed effects.
Conclusions: Together with their spontaneous commitment to cardiac and angiogenic differentiation, transplanted CDCs serve as "role models," recruiting endogenous regeneration and improving tissue resistance to ischemic stress. The contribution of the role model effect rivals or exceeds that of direct regeneration.
Figures



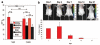

Similar articles
-
Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair.Circ Res. 2015 May 8;116(10):e81-94. doi: 10.1161/CIRCRESAHA.115.306146. Epub 2015 Mar 23. Circ Res. 2015. PMID: 25801898
-
Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model.Cardiovasc Res. 2012 Sep 1;95(4):495-506. doi: 10.1093/cvr/cvs224. Epub 2012 Aug 10. Cardiovasc Res. 2012. PMID: 22886775
-
Paracrine Engineering of Human Cardiac Stem Cells With Insulin-Like Growth Factor 1 Enhances Myocardial Repair.J Am Heart Assoc. 2015 Sep 11;4(9):e002104. doi: 10.1161/JAHA.115.002104. J Am Heart Assoc. 2015. PMID: 26363004 Free PMC article.
-
The paracrine effect: pivotal mechanism in cell-based cardiac repair.J Cardiovasc Transl Res. 2010 Dec;3(6):652-62. doi: 10.1007/s12265-010-9198-2. Epub 2010 Jun 8. J Cardiovasc Transl Res. 2010. PMID: 20559770 Review.
-
Angiogenesis after acute myocardial infarction.Cardiovasc Res. 2021 Apr 23;117(5):1257-1273. doi: 10.1093/cvr/cvaa287. Cardiovasc Res. 2021. PMID: 33063086 Review.
Cited by
-
Assessment of Myocardial Diastolic Dysfunction as a Result of Myocardial Infarction and Extracellular Matrix Regulation Disorders in the Context of Mesenchymal Stem Cell Therapy.J Clin Med. 2022 Sep 15;11(18):5430. doi: 10.3390/jcm11185430. J Clin Med. 2022. PMID: 36143077 Free PMC article. Review.
-
Macrophage migration inhibitory factor promotes cardiac stem cell proliferation and endothelial differentiation through the activation of the PI3K/Akt/mTOR and AMPK pathways.Int J Mol Med. 2016 May;37(5):1299-309. doi: 10.3892/ijmm.2016.2542. Epub 2016 Mar 29. Int J Mol Med. 2016. PMID: 27035848 Free PMC article.
-
New strategies for improving stem cell therapy in ischemic heart disease.Heart Fail Rev. 2016 Nov;21(6):737-752. doi: 10.1007/s10741-016-9576-1. Heart Fail Rev. 2016. PMID: 27459850 Review.
-
Extracellular vesicles in cardiac repair and regeneration: Beyond stem-cell-based approaches.Front Cell Dev Biol. 2022 Sep 2;10:996887. doi: 10.3389/fcell.2022.996887. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120584 Free PMC article. Review.
-
Myocardial regeneration of the failing heart.Heart Fail Rev. 2013 Nov;18(6):815-33. doi: 10.1007/s10741-012-9348-5. Heart Fail Rev. 2013. PMID: 23001638 Review.
References
-
- Rosenzweig A. Cardiac cell therapy-mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. - PubMed
-
- Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. - PubMed
-
- Guan K, Hasenfuss G. Do stem cells in the heart truly differentiate into cardiomyocytes? J Mol Cell Cardiol. 2007;43:377–387. - PubMed
ONLINE SUPPLEMENTAL REFERENCES
-
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. - PubMed
-
- Iravanian S, Nabutovsky Y, Kong CR, Saha S, Bursac N, Tung L. Functional reentry in cultured monolayers of neonatal rat cardiac cells. Am J Physiol Heart Circ Physiol. 2003;285:H449–456. - PubMed
-
- Kizana E, Ginn SL, Allen DG, Ross DL, Alexander IE. Fibroblasts can be genetically modified to produce excitable cells capable of electrical coupling. Circulation. 2005;111:394–398. - PubMed
-
- Zhang YW, Su Y, Lanning N, Gustafson M, Shinomiya N, Zhao P, Cao B, Tsarfaty G, Wang LM, Hay R, Vande Woude GF. Enhanced growth of human met-expressing xenografts in a new strain of immunocompromised mice transgenic for human hepatocyte growth factor/scatter factor. Oncogene. 2005;24:101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

