Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of DiGeorge syndrome
- PMID: 20110535
- PMCID: PMC2864540
- DOI: 10.1161/CIRCRESAHA.109.205732
Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of DiGeorge syndrome
Abstract
Rationale: Loss of Tbx1 and decrease of retinoic acid (RA) synthesis result in DiGeorge/velocardiofacial syndrome (DGS/VCFS)-like phenotypes in mouse models, including defects in septation of the outflow tract of the heart and anomalies of pharyngeal arch-derived structures including arteries of the head and neck, laryngeal-tracheal cartilage, and thymus/parathyroid. Wild-type levels of T-box transcription factor (Tbx)1 and RA signaling are required for normal pharyngeal arch artery development. Recent studies have shown that reduction of RA or loss of Tbx1 alters the contribution of second heart field (SHF) progenitor cells to the elongating heart tube.
Objective: Here we tested whether Tbx1 and the RA signaling pathway interact during the deployment of the SHF and formation of the mature aortic arch.
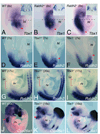
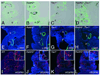
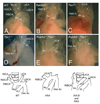
Methods and results: Molecular markers of the SHF, neural crest and smooth muscle cells, were analyzed in Raldh2;Tbx1 compound heterozygous mutants. Our results revealed that the SHF and outflow tract develop normally in Raldh2(+/-);Tbx1(+/-) embryos. However, we found that decreased levels of RA accelerate the recovery from arterial growth delay observed in Tbx1(+/-) mutant embryos. This compensation coincides with the differentiation of smooth muscle cells in the 4th pharyngeal arch arteries, and is associated with severity of neural crest cell migration defects observed in these mutants.
Conclusions: Our data suggest that differences in levels of embryonic RA may contribute to the variability in great artery anomalies observed in DGS/VCFS patients.
Figures


Comment in
-
DiGeorge syndrome, Tbx1, and retinoic acid signaling come full circle.Circ Res. 2010 Mar 5;106(4):630-2. doi: 10.1161/CIRCRESAHA.109.215319. Circ Res. 2010. PMID: 20203312 Free PMC article. No abstract available.
References
-
- Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. - PubMed
-
- Lindsay EA. Chromosomal microdeletions: dissecting del22q11 syndrome. Nat Rev Genet. 2001;2:858–868. - PubMed
-
- Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279–284. - PubMed
-
- Hu T, Yamagishi H, Maeda J, McAnally J, Yamagishi C, Srivastava D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development. 2004;131:5491–5502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

