Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo
- PMID: 20110577
- PMCID: PMC3137455
- DOI: 10.1161/ATVBAHA.109.195693
Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo
Abstract
Objective: We previously reported that a systemic liver X receptor (LXR) agonist promoted macrophage reverse-cholesterol transport (mRCT) in vivo. Because LXR are expressed in multiple tissues involved in RCT (macrophages, liver, intestine), we analyzed the effect of tissue-specific LXR agonism on mRCT.
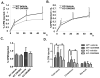
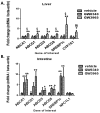
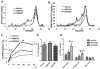
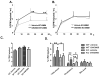
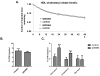
Methods and results: In initial studies, the systemic LXR agonist GW3965 failed to promote mRCT in a setting in which LXR was expressed in macrophages but not in liver or intestine. To evaluate the effect of LXR activation specifically in small intestine on mRCT, wild-type mice were treated with either intestinal-specific LXR agonist (GW6340) or systemic LXR agonist (GW3965). Both GW3965 and GW6340 significantly promoted excretion of [(3)H]-sterol in feces by 162% and 52%, respectively. To evaluate the requirement for macrophage LXR activation, we assessed the ability of GW3965 to promote mRCT in wild-type mice using primary macrophages deficient in LXR alpha/beta vs wild-type macrophages. Whereas GW3965 treatment promoted fecal excretion compared with vehicle, its overall ability to promote mRCT was significantly attenuated using LXR alpha/beta knockout macrophages.
Conclusions: We demonstrate that intestinal-specific LXR agonism promotes macrophage RCT in vivo and that macrophage LXR itself plays an important, but not predominant, role in promoting RCT in response to an LXR agonist.
Figures
References
-
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. - PubMed
-
- Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. - PubMed
-
- Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

