Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer's disease
- PMID: 20110603
- PMCID: PMC2951486
- DOI: 10.3233/JAD-2010-1262
Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer's disease
Abstract
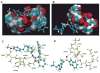
We describe the interactions of two benzimidazole derivatives, astemizole (AST) and lansoprazole (LNS), with anomalous aggregates of tau protein (neurofibrillary tangles). Interestingly, these compounds, with important medical applications in the treatment of allergies and gastrointestinal disorders respectively, specifically bind to aggregated variants of tau protein and to paired helical filaments isolated from brains of Alzheimer's disease (AD) patients. These ligands appear to be a powerful tool to tag brain-isolated tau-aggregates and heparin-induced polymers of recombinant tau. The interactions of AST and LNS with tau aggregates were assessed by classical radioligand assays, surface plasmon resonance, and bioinformatic approaches. The affinity of AST and LNS for tau aggregates was comparatively higher than that for amyloid-beta polymers according to our data. This is relevant since senile plaques are also abundant but are not pathognomonic in AD patients. Immunochemical studies on paired helical filaments from brains of AD patients and surface plasmon resonance studies confirm these findings. The capacity of these drugs to penetrate the blood-brain barrier was evaluated: i) in vitro by parallel artificial membrane permeability assay followed by experimental Log P determinations; and ii) in vivo by pharmacokinetic studies comparing distribution profiles in blood and brain of mice using HPLC/UV. Importantly, our studies indicate that the brain/blood concentration ratios for these compounds were suitable for their use as PET radiotracers. Since neurofibrillary tangles are positively correlated with cognitive impairment, we concluded that LNS and AST have a great potential in PET neuroimaing for in vivo early detection of AD and in reducing the formation of neurofibrillary tangles.
Figures




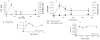
References
-
- Khachaturian ZS. An overview of Alzheimer's disease research. Am J Med. 1998;104:26S–31S. discussion 39S-42S. - PubMed
-
- Terry RD. Where in the brain does Alzheimer's disease begin? Ann Neurol. 2000;47:421. - PubMed
-
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. - PubMed
-
- Dubois B. ’Prodromal Alzheimer's disease’: a more useful concept than mild cognitive impairment? Curr Opin Neurol. 2000;13:367–369. - PubMed
-
- Dawbarn D. Neurobiology of Alzheimer's Disease. BIOS Scientific Publishers Ltd.; Oxford, UK: 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

