The H-loop in the second nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator is required for efficient chloride channel closing
- PMID: 20110677
- PMCID: PMC2844707
- DOI: 10.1159/000276549
The H-loop in the second nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator is required for efficient chloride channel closing
Abstract
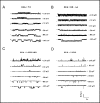
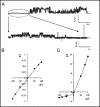
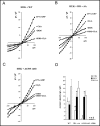
The cystic fibrosis transmembrane conductance regulator (CFTR) is an ATP-binding cassette (ABC) transporter that functions as a cAMP-activated chloride channel. The recent model of CFTR gating predicts that the ATP binding to both nucleotide-binding domains (NBD1 and NBD2) of CFTR is required for the opening of the channel, while the ATP hydrolysis at NBD2 induces subsequent channel closing. In most ABC proteins, efficient hydrolysis of ATP requires the presence of the invariant histidine residue within the H-loop located in the C-terminal part of the NBD. However, the contribution of the corresponding region (H-loop) of NBD2 to the CFTR channel gating has not been examined so far. Here we report that the alanine substitution of the conserved dipeptide HR motif (HR-->AA) in the H-loop of NBD2 leads to prolonged open states of CFTR channel, indicating that the H-loop is required for efficient channel closing. On the other hand, the HR-->AA substitution lead to the substantial decrease of CFTR-mediated current density (pA/pF) in transfected HEK 293 cells, as recorded in the whole-cell patch-clamp analysis. These results suggest that the H-loop of NBD2, apart from being required for CFTR channel closing, may be involved in regulating CFTR trafficking to the cell surface.
Copyright 2010 S. Karger AG, Basel.
Figures

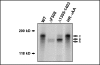
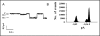

Similar articles
-
The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics.J Gen Physiol. 2006 Oct;128(4):413-22. doi: 10.1085/jgp.200609622. Epub 2006 Sep 11. J Gen Physiol. 2006. PMID: 16966475 Free PMC article.
-
Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating.J Gen Physiol. 2003 Sep;122(3):333-48. doi: 10.1085/jgp.200308798. J Gen Physiol. 2003. PMID: 12939393 Free PMC article.
-
Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. Quantitative analysis of a cyclic gating scheme.J Gen Physiol. 1999 Apr;113(4):541-54. doi: 10.1085/jgp.113.4.541. J Gen Physiol. 1999. PMID: 10102935 Free PMC article.
-
The gating of the CFTR channel.Cell Mol Life Sci. 2017 Jan;74(1):85-92. doi: 10.1007/s00018-016-2390-z. Epub 2016 Oct 1. Cell Mol Life Sci. 2017. PMID: 27696113 Free PMC article. Review.
-
ATP hydrolysis cycles and the gating of CFTR Cl- channels.Acta Physiol Scand Suppl. 1998 Aug;643:247-56. Acta Physiol Scand Suppl. 1998. PMID: 9789567 Review.
Cited by
-
Cystic fibrosis transmembrane conductance regulator (ABCC7) structure.Cold Spring Harb Perspect Med. 2013 Feb 1;3(2):a009514. doi: 10.1101/cshperspect.a009514. Cold Spring Harb Perspect Med. 2013. PMID: 23378596 Free PMC article. Review.
-
Reporting Two Novel Mutations in Two Iranian Families with Cystic Fibrosis, Molecular and Bioinformatic Analysis.Iran Biomed J. 2022 Nov 1;26(5):398-405. doi: 10.52547/ibj.3713. Iran Biomed J. 2022. PMID: 35468710 Free PMC article.
-
Disease-relevant mutations alter amino acid co-evolution networks in the second nucleotide binding domain of CFTR.PLoS One. 2020 Jan 24;15(1):e0227668. doi: 10.1371/journal.pone.0227668. eCollection 2020. PLoS One. 2020. PMID: 31978131 Free PMC article.
-
Targeting Nucleotide Binding Domain of Multidrug Resistance-associated Protein-1 (MRP1) for the Reversal of Multi Drug Resistance in Cancer.Sci Rep. 2018 Aug 10;8(1):11973. doi: 10.1038/s41598-018-30420-x. Sci Rep. 2018. PMID: 30097643 Free PMC article.
References
-
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. - PubMed
-
- Riordan The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. - PubMed
-
- Coakley RD, Stutts MJ. Function of CFTR Protein: Regulatory Functions. In: Bush A, Alton EWFW, Davies JC, Griesenbach U, Jaffe A, editors. Cystic Fibrosis in the 21st Century. vol 34. Basel: Karger; 2006. pp. 45–53.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
