Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells
- PMID: 20110858
- PMCID: PMC2844483
- DOI: 10.1097/TP.0b013e3181c9dc7d
Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells
Abstract
Background: The role of bone marrow (BM)-derived cells in pancreatic beta-cell regeneration remains unresolved. We examined whether BM-derived cells are recruited to the site of moderate pancreatic injury and contribute to beta-cell regeneration.
Methods: Low-dose streptozotocin (STZ) treatment was used to induce moderate pancreatic damage and hyperglycemia. Enhanced green fluorescent protein-positive (EGFP) BM chimeras were evaluated for beta-cell regeneration after STZ treatment.
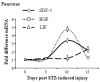
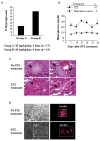
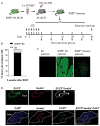
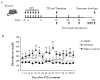
Results: To test the hypothesis that pancreatic tissue injury induces a stromal cell-derived factor (SDF)-1 gradient to chemoattract the stem cells, we evaluated the expression of mRNA for SDF-1 in damaged pancreatic tissue. SDF-1 was significantly increased in the pancreas after damage, peaking at day 10. The majority of BM cells expressing mRNA for pancreatic development markers were detected in the subpopulation of CD45/Sca-1/Lin very small embryonic-like (VSEL) cells. VSEL cells mobilized from BM to peripheral blood in response to pancreatic damage, peaking in peripheral blood at day 5, and were enriched in the pancreas 10 to 15 days after STZ treatment. To confirm a role for BM-derived cells in pancreatic beta-cell regeneration, we prepared EGFP-->B6 chimeras. In the EGFP chimeras, EGFP cells were detected around duct and islets and were positive for insulin after STZ treatment. However, STZ-induced hyperglycemia was reduced only transiently (49-77 days) after pancreatic injury.
Conclusions: These data suggest that VSEL cells are mobilized into injured pancreatic tissue and contribute to beta-cell regeneration. Transplantation of BM-derived cells improves the function of injured pancreas, although the response is not sufficient to restore sustained normoglycemia.
Figures
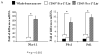
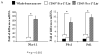

Similar articles
-
Bone marrow (BM) transplantation promotes beta-cell regeneration after acute injury through BM cell mobilization.Endocrinology. 2007 May;148(5):2006-15. doi: 10.1210/en.2006-1351. Epub 2007 Jan 25. Endocrinology. 2007. PMID: 17255204
-
Transplantation of bone marrow derived cells promotes pancreatic islet repair in diabetic mice.Biochem Biophys Res Commun. 2008 Jun 20;371(1):132-7. doi: 10.1016/j.bbrc.2008.04.033. Epub 2008 Apr 15. Biochem Biophys Res Commun. 2008. PMID: 18420028
-
Exosomes Secreted by Umbilical Cord Blood-Derived Mesenchymal Stem Cell Attenuate Diabetes in Mice.J Diabetes Res. 2021 Dec 10;2021:9534574. doi: 10.1155/2021/9534574. eCollection 2021. J Diabetes Res. 2021. PMID: 34926699 Free PMC article.
-
Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function.Stem Cells. 2010 Jan;28(1):140-51. doi: 10.1002/stem.259. Stem Cells. 2010. PMID: 19924826
-
Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration.Lab Invest. 2007 Jul;87(7):702-12. doi: 10.1038/labinvest.3700561. Epub 2007 May 7. Lab Invest. 2007. PMID: 17483848
Cited by
-
Stem cell-based therapeutic applications in retinal degenerative diseases.Stem Cell Rev Rep. 2011 Jun;7(2):434-45. doi: 10.1007/s12015-010-9192-8. Stem Cell Rev Rep. 2011. PMID: 20859770 Free PMC article. Review.
-
The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders.Prog Neurobiol. 2011 Oct;95(2):213-28. doi: 10.1016/j.pneurobio.2011.08.005. Epub 2011 Aug 30. Prog Neurobiol. 2011. PMID: 21903148 Free PMC article. Review.
-
Very small embryonic-like stem cells are involved in pancreatic regeneration and their dysfunction with age may lead to diabetes and cancer.Stem Cell Res Ther. 2015 May 15;6(1):96. doi: 10.1186/s13287-015-0084-3. Stem Cell Res Ther. 2015. PMID: 25976079 Free PMC article. Review.
-
Nutritional programming of pancreatic β-cell plasticity.World J Diabetes. 2011 Aug 15;2(8):119-26. doi: 10.4239/wjd.v2.i8.119. World J Diabetes. 2011. PMID: 21954415 Free PMC article.
-
PDX1-engineered embryonic stem cell-derived insulin producing cells regulate hyperglycemia in diabetic mice.Transplant Res. 2012 Oct 18;1(1):19. doi: 10.1186/2047-1440-1-19. Transplant Res. 2012. PMID: 23369186 Free PMC article.
References
-
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369. - PubMed
-
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775. - PubMed
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41. - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

