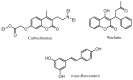
Synthesis and vasorelaxant and platelet antiaggregatory activities of a new series of 6-halo-3-phenylcoumarins
- PMID: 20110890
- PMCID: PMC6257048
- DOI: 10.3390/molecules15010270
Synthesis and vasorelaxant and platelet antiaggregatory activities of a new series of 6-halo-3-phenylcoumarins
Abstract
A series of 6-halo-3-hydroxyphenylcoumarins (resveratrol-coumarins hybrid derivatives) was synthesized in good yields by a Perkin reaction followed by hydrolysis. The new compounds were evaluated for their vasorelaxant activity in intact rat aorta rings pre-contracted with phenylephrine (PE), as well as for their inhibitory effects on platelet aggregation induced by thrombin in washed human platelets. These compounds concentration-dependently relaxed vascular smooth muscle and some of them showed a platelet antiaggregatory activity that was up to thirty times higher than that shown by trans-resveratrol and some other previously synthesized derivatives.
Figures
Similar articles
-
3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade.Molecules. 2021 Nov 8;26(21):6755. doi: 10.3390/molecules26216755. Molecules. 2021. PMID: 34771164 Free PMC article. Review.
-
Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids.Bioorg Med Chem Lett. 2006 Jan 15;16(2):257-61. doi: 10.1016/j.bmcl.2005.10.013. Epub 2005 Nov 3. Bioorg Med Chem Lett. 2006. PMID: 16275073
-
Synthesis and vasorelaxant activity of new coumarin and furocoumarin derivatives.Bioorg Med Chem Lett. 2002 Mar 11;12(5):783-6. doi: 10.1016/s0960-894x(02)00015-x. Bioorg Med Chem Lett. 2002. PMID: 11859002
-
Design and synthesis of novel biologically active thrombin receptor non-peptide mimetics based on the pharmacophoric cluster Phe/Arg/NH2 of the Ser42-Phe-Leu-Leu-Arg46 motif sequence: platelet aggregation and relaxant activities.J Med Chem. 2004 Jun 17;47(13):3338-52. doi: 10.1021/jm031080v. J Med Chem. 2004. PMID: 15189031
-
Resveratrol, a phenolic antioxidant with effects on blood platelet functions.Platelets. 2005 Aug;16(5):251-60. doi: 10.1080/09537100400020591. Platelets. 2005. PMID: 16011975 Review.
Cited by
-
3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade.Molecules. 2021 Nov 8;26(21):6755. doi: 10.3390/molecules26216755. Molecules. 2021. PMID: 34771164 Free PMC article. Review.
-
3-Phenylcoumarins as inhibitors of HIV-1 replication.Molecules. 2012 Aug 2;17(8):9245-57. doi: 10.3390/molecules17089245. Molecules. 2012. PMID: 22858844 Free PMC article.
-
Natural source, bioactivity and synthesis of 3-Arylcoumarin derivatives.J Enzyme Inhib Med Chem. 2022 Dec;37(1):1023-1042. doi: 10.1080/14756366.2022.2058499. J Enzyme Inhib Med Chem. 2022. PMID: 35438580 Free PMC article. Review.
-
Structural Insight of New Butyrylcholinesterase Inhibitors Based on Benzylbenzofuran Scaffold.Pharmaceuticals (Basel). 2022 Mar 2;15(3):304. doi: 10.3390/ph15030304. Pharmaceuticals (Basel). 2022. PMID: 35337102 Free PMC article.
-
Blocking oestradiol synthesis pathways with potent and selective coumarin derivatives.J Enzyme Inhib Med Chem. 2018 Dec;33(1):743-754. doi: 10.1080/14756366.2018.1452919. J Enzyme Inhib Med Chem. 2018. PMID: 29620427 Free PMC article.
References
-
- Fiedler V.B., Scholtholt J. Effects of carbocromene on myocardial oxygen consumption in isolated dog hearts. J. Pharmacol. Exp. Ther. 1981;217:306–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical