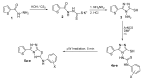Synthesis and antimicrobial activity of new 5-(2-thienyl)-1,2,4-triazoles and 5-(2-thienyl)-1,3,4-oxadiazoles and related derivatives
- PMID: 20110905
- PMCID: PMC6257108
- DOI: 10.3390/molecules15010502
Synthesis and antimicrobial activity of new 5-(2-thienyl)-1,2,4-triazoles and 5-(2-thienyl)-1,3,4-oxadiazoles and related derivatives
Abstract
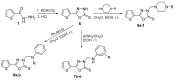
New 5-(2-thienyl)-1,2,4-triazoles and 5-(2-thienyl)-1,3,4-oxadiazoles namely, N-[3-mercapto-5-(2-thienyl)-1,2,4-triazol-4-yl]-N'-arylthioureas 4a-e, 2-arylamino-5-(2-thienyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles 5a-e, 3-arylaminomethyl-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 7a-e, 3-(N-substituted anilinomethyl)-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 8a, b and 3-(4-substituted-1-piperazinylmethyl)-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 9a-f, were prepared. The synthesized compounds were tested for in vitro activities against certain strains of Gram-positive and Gram-negative bacteria and the yeast-like pathogenic fungus Candida albicans. Compound 9a displayed marked broad spectrum antibacterial activity, while compounds 4d, 5e, 7b, 7c, 7d, 9b, 9c and 9d were highly active against the tested Gram-positive bacteria. None of the synthesized compounds were proved to be significantly active against Candida albicans.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases