Photoresponsive block copolymers containing azobenzenes and other chromophores
- PMID: 20110910
- PMCID: PMC6256985
- DOI: 10.3390/molecules15010570
Photoresponsive block copolymers containing azobenzenes and other chromophores
Abstract
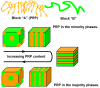
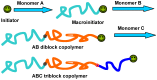
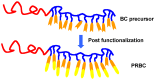
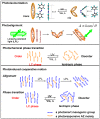
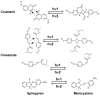
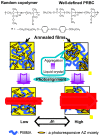
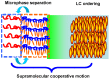
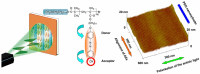
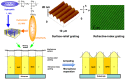
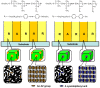
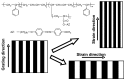
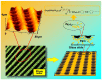
Photoresponsive block copolymers (PRBCs) containing azobenzenes and other chromophores can be easily prepared by controlled polymerization. Their photoresponsive behaviors are generally based on photoisomerization, photocrosslinking, photoalignment and photoinduced cooperative motions. When the photoactive block forms mesogenic phases upon microphase separation of PRBCs, supramolecular cooperative motion in liquid-crystalline PRBCs enables them to self-organize into hierarchical structures with photoresponsive features. This offers novel opportunities to photocontrol microphase-separated nanostructures of well-defined PRBCs and extends their diverse applications in holograms, nanotemplates, photodeformed devices and microporous films.
Figures








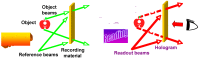

Similar articles
-
Photoalignment of Vertically Oriented Microphase Separated Lamellae in LC-LC Diblock Copolymer Thin Film.Macromol Rapid Commun. 2017 Jul;38(13). doi: 10.1002/marc.201600659. Epub 2017 Mar 24. Macromol Rapid Commun. 2017. PMID: 28338244
-
Vertical Orientation of Nanocylinders in Liquid-Crystalline Block Copolymers Directed by Light.ACS Appl Mater Interfaces. 2017 Jul 26;9(29):24864-24872. doi: 10.1021/acsami.7b06086. Epub 2017 Jul 17. ACS Appl Mater Interfaces. 2017. PMID: 28670902
-
Novel Photoresponsive Linear, Graft, and Comb-Like Copolymers with Azobenzene Chromophores in the Main-Chain and/or Side-Chain: Facile One-Pot Synthesis and Photoresponse Properties.Macromol Rapid Commun. 2015 Sep;36(17):1578-84. doi: 10.1002/marc.201500230. Epub 2015 Jun 22. Macromol Rapid Commun. 2015. PMID: 26098645
-
Meso- and microscopic motions in photoresponsive liquid crystalline polymer films.Macromol Rapid Commun. 2014 Feb;35(3):271-90. doi: 10.1002/marc.201300763. Epub 2013 Dec 17. Macromol Rapid Commun. 2014. PMID: 24343758 Review.
-
Polymeric nanostructures based on azobenzene and their biomedical applications: synthesis, self-assembly and stimuli-responsiveness.Org Biomol Chem. 2022 Jan 26;20(4):749-767. doi: 10.1039/d1ob01823j. Org Biomol Chem. 2022. PMID: 34908082 Review.
Cited by
-
An Electron Spin Resonance Study Comparing Nanometer-Nanosecond Dynamics in Diblock Copolymers and Their Poly(methyl Methacrylate) Binary Blends.Polymers (Basel). 2023 Oct 23;15(20):4195. doi: 10.3390/polym15204195. Polymers (Basel). 2023. PMID: 37896439 Free PMC article.
-
Photoresponse of new azo pyridine functionalized poly(2-hydroxyethyl methacrylate-co-methyl methacrylate).Sci Rep. 2024 Apr 20;14(1):9078. doi: 10.1038/s41598-024-59704-1. Sci Rep. 2024. PMID: 38643277 Free PMC article.
-
Visible Light-Driven Alkyne-Grafted Ethylene-Bridged Azobenzene Chromophores for Photothermal Utilization.Molecules. 2022 May 20;27(10):3296. doi: 10.3390/molecules27103296. Molecules. 2022. PMID: 35630773 Free PMC article.
-
Correlation between Nonlinear Optical Effects and Structural Features of Aurone-Based Methacrylic Polymeric Thin Films.Materials (Basel). 2022 Sep 1;15(17):6076. doi: 10.3390/ma15176076. Materials (Basel). 2022. PMID: 36079459 Free PMC article.
-
Azobenzene as a photoswitchable mechanophore.Nat Chem. 2024 Mar;16(3):446-455. doi: 10.1038/s41557-023-01389-6. Epub 2023 Dec 5. Nat Chem. 2024. PMID: 38052946
References
-
- Kumar G., Neckers D. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989;89:1915–1925. doi: 10.1021/cr00098a012. - DOI
-
- Xie S., Natansohn A., Rochon P. Recent developments in aromatic azo polymers research. Chem. Mater. 1993;5:403–411. doi: 10.1021/cm00028a003. - DOI
-
- Viswanathan N., Kim D., Bian S., Williams J., Liu W., Li L., Samuelson L., Kumar J., Tripathy S. Surface relief structures on azo polymer films. J. Mater. Chem. 1999;9:1941–1955. doi: 10.1039/a902424g. - DOI
-
- Hvilsted S., Ramanujam P.S. The azobenzene optical storage puzzle-demands on the polymer scaffold? Monatshefte fur Chemie. 2001;132:43–51. doi: 10.1007/s007060170143. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

