Towards building a chromosome segregation machine
- PMID: 20110988
- PMCID: PMC2879044
- DOI: 10.1038/nature08912
Towards building a chromosome segregation machine
Abstract
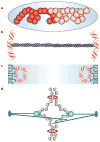
All organisms, from bacteria to humans, face the daunting task of replicating, packaging and segregating up to two metres (about 6 x 10(9) base pairs) of DNA when each cell divides. This task is carried out up to a trillion times during the development of a human from a single fertilized cell. The strategy by which DNA is replicated is now well understood. But when it comes to packaging and segregating a genome, the mechanisms are only beginning to be understood and are often as variable as the organisms in which they are studied.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

