Structure of a bacterial homologue of vitamin K epoxide reductase
- PMID: 20110994
- PMCID: PMC2919313
- DOI: 10.1038/nature08720
Structure of a bacterial homologue of vitamin K epoxide reductase
Abstract
Vitamin K epoxide reductase (VKOR) generates vitamin K hydroquinone to sustain gamma-carboxylation of many blood coagulation factors. Here, we report the 3.6 A crystal structure of a bacterial homologue of VKOR from Synechococcus sp. The structure shows VKOR in complex with its naturally fused redox partner, a thioredoxin-like domain, and corresponds to an arrested state of electron transfer. The catalytic core of VKOR is a four transmembrane helix bundle that surrounds a quinone, connected through an additional transmembrane segment with the periplasmic thioredoxin-like domain. We propose a pathway for how VKOR uses electrons from cysteines of newly synthesized proteins to reduce a quinone, a mechanism confirmed by in vitro reconstitution of vitamin K-dependent disulphide bridge formation. Our results have implications for the mechanism of the mammalian VKOR and explain how mutations can cause resistance to the VKOR inhibitor warfarin, the most commonly used oral anticoagulant.
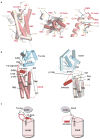
Figures





Similar articles
-
Vitamin K epoxide reductase prefers ER membrane-anchored thioredoxin-like redox partners.Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15027-32. doi: 10.1073/pnas.1009972107. Epub 2010 Aug 9. Proc Natl Acad Sci U S A. 2010. PMID: 20696932 Free PMC article.
-
Structures of an intramembrane vitamin K epoxide reductase homolog reveal control mechanisms for electron transfer.Nat Commun. 2014;5:3110. doi: 10.1038/ncomms4110. Nat Commun. 2014. PMID: 24477003 Free PMC article.
-
Human vitamin K epoxide reductase and its bacterial homologue have different membrane topologies and reaction mechanisms.J Biol Chem. 2012 Oct 5;287(41):33945-55. doi: 10.1074/jbc.M112.402941. Epub 2012 Aug 24. J Biol Chem. 2012. PMID: 22923610 Free PMC article.
-
Structure and function of vitamin K epoxide reductase.Vitam Horm. 2008;78:103-30. doi: 10.1016/S0083-6729(07)00006-4. Vitam Horm. 2008. PMID: 18374192 Review.
-
Structural and cellular basis of vitamin K antagonism.J Thromb Haemost. 2022 Sep;20(9):1971-1983. doi: 10.1111/jth.15800. Epub 2022 Jul 12. J Thromb Haemost. 2022. PMID: 35748323 Review.
Cited by
-
Free-Radical Membrane Protein Footprinting by Photolysis of Perfluoroisopropyl Iodide Partitioned to Detergent Micelle by Sonication.Angew Chem Int Ed Engl. 2021 Apr 12;60(16):8867-8873. doi: 10.1002/anie.202014096. Epub 2021 Mar 10. Angew Chem Int Ed Engl. 2021. PMID: 33751812 Free PMC article.
-
Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum redox homeostasis.J Biol Chem. 2014 Aug 15;289(33):23086-23096. doi: 10.1074/jbc.M114.570911. Epub 2014 Jul 2. J Biol Chem. 2014. PMID: 24990953 Free PMC article.
-
Intramembrane Thiol Oxidoreductases: Evolutionary Convergence and Structural Controversy.Biochemistry. 2018 Jan 23;57(3):258-266. doi: 10.1021/acs.biochem.7b00876. Epub 2017 Nov 7. Biochemistry. 2018. PMID: 29064673 Free PMC article.
-
Vitamin K epoxide reductase and its paralogous enzyme have different structures and functions.Sci Rep. 2017 Dec 15;7(1):17632. doi: 10.1038/s41598-017-18008-3. Sci Rep. 2017. PMID: 29247216 Free PMC article.
-
Warfarin traps human vitamin K epoxide reductase in an intermediate state during electron transfer.Nat Struct Mol Biol. 2017 Jan;24(1):69-76. doi: 10.1038/nsmb.3333. Epub 2016 Dec 5. Nat Struct Mol Biol. 2017. PMID: 27918545 Free PMC article.
References
-
- Oldenburg J, Marinova M, Muller-Reible C, Watzka M. The vitamin K cycle. Vitam Horm. 2008;78:35–62. - PubMed
-
- Tie JK, Stafford DW. Structure and function of vitamin K epoxide reductase. Vitam Horm. 2008;78:103–30. - PubMed
-
- Stenflo J, Suttie JW. Vitamin K-dependent formation of gamma-carboxyglutamic acid. Annu Rev Biochem. 1977;46:157–72. - PubMed
-
- Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–808. - PubMed
-
- Wajih N, Hutson SM, Wallin R. Disulfide-dependent protein folding is linked to operation of the vitamin K cycle in the endoplasmic reticulum. A protein disulfide isomerase-VKORC1 redox enzyme complex appears to be responsible for vitamin K1 2,3-epoxide reduction. J Biol Chem. 2007;282:2626–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

