Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity
- PMID: 20111004
- PMCID: PMC2845272
- DOI: 10.1038/emboj.2010.1
Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity
Abstract
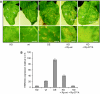
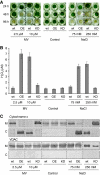
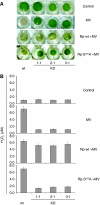
Caspases are cysteine-dependent proteases and are important components of animal apoptosis. They introduce specific breaks after aspartate residues in a number of cellular proteins mediating programmed cell death (PCD). Plants encode only distant homologues of caspases, the metacaspases that are involved in PCD, but do not possess caspase-specific proteolytic activity. Nevertheless, plants do display caspase-like activities indicating that enzymes structurally distinct from classical caspases may operate as caspase-like proteases. Here, we report the identification and characterisation of a novel PCD-related subtilisin-like protease from tobacco and rice named phytaspase (plant aspartate-specific protease) that possesses caspase specificity distinct from that of other known caspase-like proteases. We provide evidence that phytaspase is synthesised as a proenzyme, which is autocatalytically processed to generate the mature enzyme. Overexpression and silencing of the phytaspase gene showed that phytaspase is essential for PCD-related responses to tobacco mosaic virus and abiotic stresses. Phytaspase is constitutively secreted into the apoplast before PCD, but unexpectedly is re-imported into the cell during PCD providing insights into how phytaspase operates.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


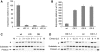

References
-
- Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Internat 11: 36–42
-
- Bassham DC (2007) Plant autophagy-more than a starvation response. Curr Opin Plant Biol 10: 587–593 - PubMed
-
- Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15: 725–731 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

